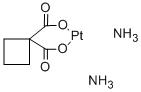
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read for reference 1 minute a day, give you professional "talking information" in the tumor circle! (If you need the original text of the literature, you can add to the editor's WeChat yxj_oncology to obtain) Key points: no matter whether Helicobacter pylori is eradicated or not, proton pump inhibitors increase the risk of gastric cancer.
Ovariectomy Cancer: Aspirin and ibuprofen can reduce the risk of occurrence and recurrence of advanced colorectal adenomas.
New drug: ADC drug Gosarturumab will be included in the application for priority review in China to treat triple-negative adult breast cancer 01Gut: No matter Whether Helicobacter pylori is eradicated or not, proton pump inhibitors all increase the risk of gastric cancer.
Gut published a cohort study online recently, showing that regardless of the status of Helicobacter pylori infection, the use of proton pump inhibitors (PPI) is related to the occurrence of gastric cancer.
Article release screenshots This study evaluated the incidence of gastric cancer after 1 year of use of PPI.
A total of 11741 patients with PPI used and non-PPI matched by propensity scores were included.
With a median follow-up time of 4.
3 years, the incidence of gastric cancer in PPI patients increased by 1.
37 times (PPI≥30 days vs non-PPI: 118/51813 person-years vs 40/49729 person-years, HR 2.
37, p=0.
001).
The incidence of gastric cancer has a parallel trend with the duration of PPI use.
In patients with Helicobacter pylori eradication, PPI use ≥180 days was significantly associated with the occurrence of gastric cancer (PPI≥180 days vs non-PPI: 30/12470 person-years vs 9/7814 person-years; HR 2.
22, p=0.
036).
The above results indicate that the use of PPI is related to the occurrence of gastric cancer and is not affected by the eradication of Helicobacter pylori.
Long-term use of PPI should be cautious in areas with high risk of gastric cancer.
02Cancers: Increased plasma protein markers in healthy BRCA1/2 carriers may suggest ovariectomy.
A few days ago, Cancers published a study online showing that plasma SPARC and THBS1 protein concentrations may be correlated with BRCA1/2 mutation carriers in ovarian cancer (OC) Risk related.
Screenshot of article release.
The study collected 20 ovarian cancer patients (10 BRCA1/2 wild-type patients and 10 BRCA1/2 mutant patients) and 20 healthy subjects (10 BRCA1/2 wild-type and 10 BRCA1 /2 mutant) plasma specimens.
The analysis found that the plasma SPARC and THBS1 concentrations of healthy BRCA1/2 carriers were lower than those of BRCA1/2 mutant ovarian cancer patients.
If the plasma SPARC concentration of healthy BRCA1/2 carriers exceeds 337.
35 ng/mL or the plasma THBS1 concentration exceeds 65.
28 μg/mL, ovariectomy may be recommended.
Clinical management methods for healthy BRCA1/2 carriers 03Cancer: Aspirin and ibuprofen can reduce the risk of occurrence and recurrence of advanced colorectal adenomas.
Recently, the results of a large prospective study published online by Cancer showed that both aspirin and ibuprofen Helps reduce the risk of the occurrence, recurrence and progression of advanced colorectal adenoma in the elderly.
Screenshot of the article released.
The study prospectively evaluated the use of aspirin and ibuprofen in the prostate, lung, colorectal, and ovarian (PLCO) tumor screening program, as well as distal adenomas (1221 cases), recurrent adenomas (862 cases) and Correlation between colorectal cancer (CRC; 2826 cases).
The follow-up time was as long as 13 years.
The results showed that the use of ibuprofen (≥30 tablets/month vs<4 tablets/month) was associated with a significant reduction in the risk of new adenomas (OR 0.
76; Ptrend=0.
04), especially advanced adenomas (OR 0.
48; Ptrend= 0.
005).
For patients with adenomas found in previous screening, the use of aspirin (≥30 tablets/month vs.
<4 tablets/month) was significantly associated with the risk of advanced recurrent adenoma (OR 0.
56; Ptrend=0.
006). The use of aspirin (HR 0.
81; Ptrend <0.
001) and ibuprofen (HR 0.
81; Ptrend=0.
003) were associated with a decrease in the risk of CRC.
04 New drug: The ADC drug Gosartuzumab is planned to be included in the priority review for the treatment of triple-negative adult breast cancer.
On May 12, the information on the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China showed that, The listing application for the antibody-conjugated drug (ADC) sacituzumab govitecan submitted by Genting Xinyao is planned to be included in the priority review.
Press release screenshots On April 22, 2020, the U.
S.
Food and Drug Administration (FDA) accelerated the approval of gosartuzumab for the treatment of patients with treated triple-negative breast cancer.
In its clinical trials, gosartuzumab achieved an overall response rate (ORR) of 33.
3%, and the median duration of response was 7.
7 months.
Among the patients who had remission, 55.
6% had remission lasting more than 6 months, and 16.
7% had remission lasting more than 12 months.
This time, gosartuzumab was included in the priority review and approval procedure according to the priority review scope "(5) Drugs that meet the conditions for approval".
References: [1]Seung In Seo,Chan Hyuk Park,Seng Chan You,et al.
Association between proton pump inhibitor use and gastric cancer:a population-based cohort study using two different types of nationwide databases in Korea.
published on May 11,2021.
doi:http://dx.
doi.
org/10.
1136/gutjnl-2020-323845.
[2]Hee-Sung Ahn,Jung Yoon Ho,Jiyoung Yu,et al.
Plasma Protein Biomarkers Associated with Higher Ovarian Cancer Risk in BRCA1/2 Carriers.
published on May 11,2021.
doi:https://doi.
org/10.
3390/cancers13102300.
[3]Kenechukwu Chudy-Onwugaje,Wen-Yi Huang,L.
Joseph Su,et al.
Aspirin ,ibuprofen,and reduced risk of advanced colorectal adenoma incidence and recurrence and colorectal cancer in the PLCO Cancer Screening Trial.
published on May 11,2021.
doi:https://doi.
org/10.
1002/cncr.
33623.
[4]http ://www.
cde.
org.
cn/news.
do?method=changePage&pageName=service&frameStr=25#.
Ovariectomy Cancer: Aspirin and ibuprofen can reduce the risk of occurrence and recurrence of advanced colorectal adenomas.
New drug: ADC drug Gosarturumab will be included in the application for priority review in China to treat triple-negative adult breast cancer 01Gut: No matter Whether Helicobacter pylori is eradicated or not, proton pump inhibitors all increase the risk of gastric cancer.
Gut published a cohort study online recently, showing that regardless of the status of Helicobacter pylori infection, the use of proton pump inhibitors (PPI) is related to the occurrence of gastric cancer.
Article release screenshots This study evaluated the incidence of gastric cancer after 1 year of use of PPI.
A total of 11741 patients with PPI used and non-PPI matched by propensity scores were included.
With a median follow-up time of 4.
3 years, the incidence of gastric cancer in PPI patients increased by 1.
37 times (PPI≥30 days vs non-PPI: 118/51813 person-years vs 40/49729 person-years, HR 2.
37, p=0.
001).
The incidence of gastric cancer has a parallel trend with the duration of PPI use.
In patients with Helicobacter pylori eradication, PPI use ≥180 days was significantly associated with the occurrence of gastric cancer (PPI≥180 days vs non-PPI: 30/12470 person-years vs 9/7814 person-years; HR 2.
22, p=0.
036).
The above results indicate that the use of PPI is related to the occurrence of gastric cancer and is not affected by the eradication of Helicobacter pylori.
Long-term use of PPI should be cautious in areas with high risk of gastric cancer.
02Cancers: Increased plasma protein markers in healthy BRCA1/2 carriers may suggest ovariectomy.
A few days ago, Cancers published a study online showing that plasma SPARC and THBS1 protein concentrations may be correlated with BRCA1/2 mutation carriers in ovarian cancer (OC) Risk related.
Screenshot of article release.
The study collected 20 ovarian cancer patients (10 BRCA1/2 wild-type patients and 10 BRCA1/2 mutant patients) and 20 healthy subjects (10 BRCA1/2 wild-type and 10 BRCA1 /2 mutant) plasma specimens.
The analysis found that the plasma SPARC and THBS1 concentrations of healthy BRCA1/2 carriers were lower than those of BRCA1/2 mutant ovarian cancer patients.
If the plasma SPARC concentration of healthy BRCA1/2 carriers exceeds 337.
35 ng/mL or the plasma THBS1 concentration exceeds 65.
28 μg/mL, ovariectomy may be recommended.
Clinical management methods for healthy BRCA1/2 carriers 03Cancer: Aspirin and ibuprofen can reduce the risk of occurrence and recurrence of advanced colorectal adenomas.
Recently, the results of a large prospective study published online by Cancer showed that both aspirin and ibuprofen Helps reduce the risk of the occurrence, recurrence and progression of advanced colorectal adenoma in the elderly.
Screenshot of the article released.
The study prospectively evaluated the use of aspirin and ibuprofen in the prostate, lung, colorectal, and ovarian (PLCO) tumor screening program, as well as distal adenomas (1221 cases), recurrent adenomas (862 cases) and Correlation between colorectal cancer (CRC; 2826 cases).
The follow-up time was as long as 13 years.
The results showed that the use of ibuprofen (≥30 tablets/month vs<4 tablets/month) was associated with a significant reduction in the risk of new adenomas (OR 0.
76; Ptrend=0.
04), especially advanced adenomas (OR 0.
48; Ptrend= 0.
005).
For patients with adenomas found in previous screening, the use of aspirin (≥30 tablets/month vs.
<4 tablets/month) was significantly associated with the risk of advanced recurrent adenoma (OR 0.
56; Ptrend=0.
006). The use of aspirin (HR 0.
81; Ptrend <0.
001) and ibuprofen (HR 0.
81; Ptrend=0.
003) were associated with a decrease in the risk of CRC.
04 New drug: The ADC drug Gosartuzumab is planned to be included in the priority review for the treatment of triple-negative adult breast cancer.
On May 12, the information on the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China showed that, The listing application for the antibody-conjugated drug (ADC) sacituzumab govitecan submitted by Genting Xinyao is planned to be included in the priority review.
Press release screenshots On April 22, 2020, the U.
S.
Food and Drug Administration (FDA) accelerated the approval of gosartuzumab for the treatment of patients with treated triple-negative breast cancer.
In its clinical trials, gosartuzumab achieved an overall response rate (ORR) of 33.
3%, and the median duration of response was 7.
7 months.
Among the patients who had remission, 55.
6% had remission lasting more than 6 months, and 16.
7% had remission lasting more than 12 months.
This time, gosartuzumab was included in the priority review and approval procedure according to the priority review scope "(5) Drugs that meet the conditions for approval".
References: [1]Seung In Seo,Chan Hyuk Park,Seng Chan You,et al.
Association between proton pump inhibitor use and gastric cancer:a population-based cohort study using two different types of nationwide databases in Korea.
published on May 11,2021.
doi:http://dx.
doi.
org/10.
1136/gutjnl-2020-323845.
[2]Hee-Sung Ahn,Jung Yoon Ho,Jiyoung Yu,et al.
Plasma Protein Biomarkers Associated with Higher Ovarian Cancer Risk in BRCA1/2 Carriers.
published on May 11,2021.
doi:https://doi.
org/10.
3390/cancers13102300.
[3]Kenechukwu Chudy-Onwugaje,Wen-Yi Huang,L.
Joseph Su,et al.
Aspirin ,ibuprofen,and reduced risk of advanced colorectal adenoma incidence and recurrence and colorectal cancer in the PLCO Cancer Screening Trial.
published on May 11,2021.
doi:https://doi.
org/10.
1002/cncr.
33623.
[4]http ://www.
cde.
org.
cn/news.
do?method=changePage&pageName=service&frameStr=25#.