-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
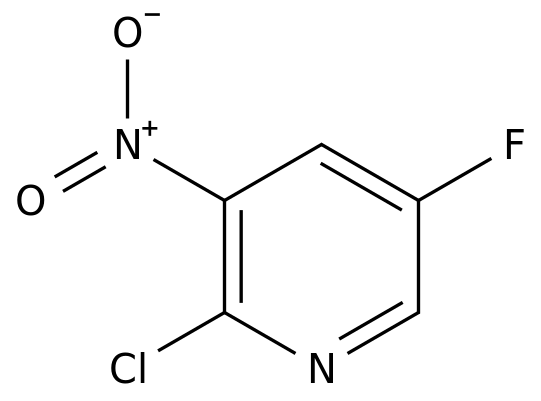
On October 19, NMPA issued a batch, Chengdu Bett 4 class of generic drug "Fumarate propofol to Nofowe tablets" was approved for the market, at the same time as through the consistent evaluation, become the first imitation in China.
Tenofovir alafenemide fumarate, also known as phosphate novovir/propofol novovir, is a new nucleoside retrovirase inhibitor (NRTI) developed by Gilead to treat patients with chronic hepatitis B (HBV) infections associated with reparational liver disease.
the drug was first approved in the U.S. in November 2016 under the name Vemlidy, and has since been approved in Japan, the European Union and Canada.
in November 2018, the original research was approved for import in China for the treatment of adults and adolescents (over 12 years of age, weighing at least 35 kg) HBV infection, the product name Welid.
TAF is the second FDA-approved TDF prebiotic drug with anti-HBV and human immunodeficiency virus (HIV) after TDF and is considered the strongest hepatitis B drug of all time.
the drug, while maintaining a high viral suppression rate, found no drug resistance, with low resistance, almost no renal toxicity, better bone safety, one-tenth of a dose can achieve TDF equivalent ability advantage.
the listing, Vemlidy has become a new force in Gilead's hepatitis B sector, with sales of $488 million in 2019, according to Gilead's 2019 results.
expected Vemlidy to achieve $1 billion in revenue by 2022 as the U.S. market penetrates and the Chinese market pulls in.
at home, Willida is negotiating to enter the national health insurance system in 2019.
it is understood that the price of a bottle of TAF (30 tablets) is $540, significantly lower than the previous $1,180, but this is still a significant burden for patients who need long-term medication.
it's worth noting, however, that Mylan was licensed by Gilead in 2017 to produce a replica TAF, which is understood to cost around 200, much lower than the current domestic TAF.
applied for a patent for tAF compounds through the PCT channel in 2001 and protects compounds worldwide under the international public name WO0208241A2.
Currently, TAF's compound patents are licensed in China, Europe, Japan, the United States, etc., and their patents expire as early as July 2021 in China, Europe and Japan, while patents in the United States are granted a 291-day extension and the patent protection period for compounds is extended until May 2022.
, Gilead has filed patents for TAF for salts, preparation methods, co-medications, preparations, etc.
with the expiry of patents, there are now a number of domestic enterprises began to lay out the TAF generic drug market.
with insight database, in addition to Chengdu Bett, 15 domestic companies have submitted applications for listing, and another 14 are in BE trials, which can be described as highly competitive.
among them, Zhengda Tianqing first submitted a listing application, followed by Qingfeng Pharmaceuticals, which filed a listing application under Class 3 generic drugs before the original TAF was approved in China.
, however, the third newspaper listed in Chengdu Beit anti-super-positive Tianqing and Qingfeng pharmaceutical industry, successfully obtained the first imitation of the species.
from: Insight Database ()