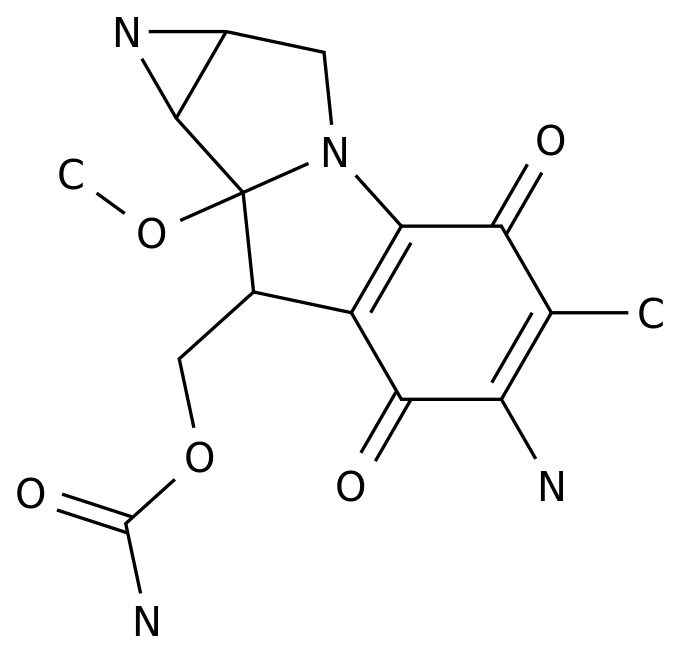
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read for reference.
Patients with prostate cancer with multiple bone metastases who have been treated with apatamide + ADT for 1 month have a rapid PSA decline, and the effect is significant.
According to the medical history, the patient was 68 years old and was admitted to the hospital on December 27, 2020 because of "physical examination found that the prostate specific antigen (PSA) was elevated for 4 days".
History of present illness: back pain, obvious symptoms after fatigue, but the patient did not pay attention.
During physical examination 4 days ago, PSA was found to be elevated, no obvious symptoms of dysuria, no frequent urination, urgency, pain, gross hematuria, waiting to urinate, hesitancy in urination, thinning of urine line, urine dripping, feeling of incomplete urination, nocturia Increased, without difficulty in defecation, without edema of both lower extremities, lymphadenopathy, anemia, bone pain, pathological fracture, and paraplegia.
The patient went to the hospital.
PET-CT examination showed that: considering prostate cancer, multiple bone metastases in the thoracic 10-lumbosacral spine and most of the bones of the pelvis, prostate tumor marker PSA 548.
98 ng/ml, no treatment.
Recently, the above-mentioned uncomfortable symptoms have increased significantly, and further treatment is required.
Past history: Hypertension for 6 years, regular medication on weekdays, and self-report of good blood pressure control.
He denied history of trauma surgery, food and drug allergy, and history of blood transfusion.
Personal history: Smoking, 15 cigarettes per day on average, no smoking cessation, no drinking habits.
Family history: No abnormalities.
Admission to the hospital for digital rectal examination: the surface of the prostate is rough and uneven, with hard nodules.
Biochemical examination (December 27, 2020): The absolute value of basophils, the percentage of basophils increased, the red blood cell count decreased, and the erythrocyte sedimentation rate increased.
The blood glucose was 7.
83mmol/L, the alkaline phosphatase (ALP) was 316 IU/L, and the estimated glomerular filtration rate (eGFR) was 68.
8ml/min/1.
73m2.
PSA 549 ng/ml; free prostate specific antigen (fPSA) 48.
4 ng/ml; fPSA/PSA (f/t) 8.
8%.
Prostate MR (December 29, 2020): mild prostate hyperplasia, prostate cancer, involving the right seminal vesicles and adjacent rectum, a few enlarged lymph nodes in the groin area; multiple bone metastases in the pelvis.
Figure 1.
MR needle biopsy of the prostate on December 29, 2020: A needle biopsy of prostate tissue was performed on December 29, 2020.
The pathology was prostate acinar carcinoma.
Figure 2.
Needle biopsy results.
Whole body bone tomography (January 5, 2021): 9 thoracic vertebrae (mild), thoracic 10-lumbar 3 vertebral bodies, lumbar 4, 5 vertebral body (mild), sacral vertebrae (Multiple locations), bilateral acetabulum, bilateral pubis, and left ischial bone are metabolically active, consider multiple bone metastases.
No clear bone metastases were found on the rest of the whole body bone imaging.
Figure 3.
2021-1-5 imaging diagnosis results of prostate acinar adenocarcinoma.
After treatment, apatamide + androgen deprivation therapy (ADT) treatment was started on January 6, 2021, and PSA was reviewed on February 4, 2021, which dropped to 7.
67 ng/ml and fPSA 1.
02 ng/ml.
Continue the apatamide + ADT treatment, until March 4, 2021, PSA continued to drop to 1.
45 ng/ml, fPSA 0.
42 ng/ml, and testosterone (Testo) 0.
09 ng/ml.
As of April 29, 2021, the patient's PSA continued to drop to 0.
32 ng/ml and fPSA 0.
10 ng/ml.
At the last follow-up (May 27, 2021), PSA dropped to 0.
21 ng/ml.
Figure 4.
PSA curve of patients treated with apatamide + ADT for 3 months, ECT examination showed: left acetabular anterior column, left upper and lower branches of pubic bone, left ilium (mild) bone metabolism is active, consider bone metastasis ; The sixth posterior rib on the right, the right acetabulum, the right suprapubic branch, and the left ischia have mild bone metabolism, except for possible bone metastasis; lumbar degeneration; the rest of the whole body bone imaging shows no clear bone metastasis .
Profile of the doctor who provided the caseProfessor Chen Dingji, Director of the Subspecialty of Prostate Tumor of Urology, Zhujiang Hospital, Southern Medical University, Deputy Chief Physician, Master's Tutor, Doctor of Medicine, Member of the Communication Group of the Youth Committee of the Urology Branch of the Chinese Medical Association Member of the Youth Committee of Guangdong Urology Branch Guangdong Member of the Standing Committee of the Urology Professional Committee of the Provincial Medical Education Association Member of the Urinary Control Group of the Urology Branch of the Guangdong Medical Association Member of the Prostate Cancer Multidisciplinary Coordination Group of the Urogenital Tumor Professional Committee of the Guangdong Anti-Cancer Association Member of the Prostate Cancer Multidisciplinary Collaboration Group 2010-2011 went to Malmö Hospital, Sweden The clinical research center conducted a 1-year case analysis of prostate cancer diagnosis and treatment related research.
This patient initially presented with low back pain but did not pay attention to it.
Later, the PSA was found to be elevated during physical examination and came to the hospital for treatment.
After a comprehensive examination, he was diagnosed with prostate cancer with multiple bone metastases.
Compared with non-metastatic patients, the 5-year survival rate of prostate cancer patients with distant metastasis is significantly lower, and the progression-free survival period is only half of that of non-metastatic patients [1,2].
How to delay disease progression and prolong patient survival is the main treatment goal of metastatic hormone-sensitive prostate cancer (mHSPC) [3].
Androgen receptor (AR) inhibitors are important therapeutic drugs for prostate cancer, but traditional AR inhibitors (such as bicalutamide) have limited benefit to patients compared with ADT treatment, and long-term use can make it inhibit AR The effect turns into an agonistic effect, which promotes the proliferation of prostate cancer cells [4-7].
The latest international guidelines no longer actively recommend its use for the treatment of mHSPC [8].
The new generation of AR inhibitor apatamide has better binding affinity to AR and better anti-tumor activity.
The TITAN study shows that the combination of apatamide combined with ADT for bone metastasis mHSPC reduces the risk of death by 50% [9].
Moreover, the incidence of overall adverse events, grade 3-4 adverse events and serious adverse events in the treatment of apatamide combined with ADT is comparable to that of the placebo group.
Long-term use does not increase the incidence of adverse events and is well tolerated.
This case was treated with apatamide+ADT, which demonstrated the rapid and profound effect of apatamide+ADT in reducing PSA levels in clinical studies.
The initial effect is expected to be transformed into prolonged overall survival.
Experts comment 1 The bone is a common metastatic site of prostate cancer, and it has a certain impact on the quality of life of patients.
For prostate cancer patients with bone metastases, how to prevent and treat bone metastases and bone-related events is particularly important while actively treating the primary lesions.
In this case of mHSPC with multiple bone metastases, the treatment plan of apatamide + ADT was used, which was in line with the recommendations of domestic and foreign guidelines.
According to the TITAN study, apatamide+ADT can significantly improve the overall survival (OS) and imaging progression-free survival (rPFS) of patients with mHSPC with multiple bone metastases.
At present, apatamide has been approved in the country for the treatment of adult patients with mHSPC.
As the clinical application of apatamide becomes more and more extensive, it is believed that there will be more information about apatamide in the real world in China in the future.
The publication of localized evidence brings more evidence-based medicine evidence for domestic prostate cancer patients and guides clinical medication.
Commentary on 1Professor Wang Shuo, Deputy Director of Department of Urology, The First Affiliated Hospital of Zhejiang University Director of Minimally Invasive Center of Urology, The First Affiliated Hospital of Zhejiang University Member of the Robotics Group and Deputy Secretary-General of the Urology Branch of the Chinese Medical Association Chairman and expert comment 2 The PSA level of the patient in this case was 549 ng/ml when he was admitted to the hospital, and he was initially given apatamide + ADT treatment.
One month later, the PSA level of the patient dropped rapidly to 7.
67 ng/ml, a drop of 98.
6%; as of 5 At the monthly follow-up, PSA dropped to 0.
21 ng/ml, a decrease of 99.
9% from the initial period.
Clinical practice has shown that PSA levels are closely related to the prognosis of patients.
The greater the reduction of PSA in the initial treatment stage, the longer the progression-free survival time of mHSPC patients; the longer the time it takes for mHSPC to progress to metastatic castration-resistant prostate cancer (mCRPC), the total number of patients The better the lifetime.
The TITAN study of apatamide shows that mHSPC patients with traditional endocrine therapy will progress to the mCRPC stage in "less than 1 year".
The median follow-up of the apatamide regimen for nearly 4 years has not reached the median value, which reduces the risk of mHSPC patients entering mCRPC.
The risk reaches 66%, which greatly prolongs the time for mHSPC to progress to mCRPC, and significantly improves the overall survival benefit of patients.
The consensus of experts in my country pointed out: The treatment goal of mHSPC is to delay disease progression and prolong survival time.
Apataamide can rapidly reduce PSA levels and delay the progression time of mHSPC, and is a good choice for initial treatment of mHSPC patients.
Reviewer 2 Introduction Professor Ding Guoqing, deputy director of the Department of Urology, Run Run Run Run Shaw Hospital, Zhejiang University School of Medicine, and Chief Physician Deputy Secretary of the Party Committee of the Run Run Run Run Hospital Affiliated to Zhejiang University School of Medicine, Member of the Standing Committee of the Urology Branch of Zhejiang Medical Association, deputy of the Minimally Invasive Group Team Leader Zhejiang Provincial Medical Association Urogenital Tumor Special Committee Deputy Chairman Zhejiang Medical Doctor Association Minimally Invasive Surgery Special Committee Member Zhejiang Anti-Cancer Association Urogenital Tumor Special Committee Deputy Chairman Zhejiang Rehabilitation Medicine Association Urology and Andrology Rehabilitation The deputy chairperson of the special committee, please pay attention to the wonderful live broadcast of the CUA clinical research training class: Reference: [1].
Siegel RL, et al.
Cancer statistics, 2018 [J].
CA: A Cancer Journal For Clinicians.
2018; 68( suppl 12): 277-300.
[2].
Ross RW, Xie WL, Regan MM, et al.
Efficacy ofandrogen deprivation therapy (ADT) in patients with advanced prostate[J].
Cancer, 2008, 112(6): 1247 -53.
[3].
Chinese Association of Urology, Chinese Association of Prostate Cancer.
Chinese Expert Consensus on Chemotherapy of Metastatic Prostate Cancer (2019 Edition)[J].
Chinese Journal of Urology,2019,40(10):721- 725.
[4].
Veldscholte J, Berrevoets CA, Ris-Stalpers C, etal.
The androgen receptor in LNCaP cells contains a mutation in the ligandbinding domain which affects steroid binding characteristics and response to antiandrogens.
The Journal of Steroid Biochemistry and Molecular Biology.
1992;41(3-8):665-669.
[5].
Liu Jun, Hu Weilie, Song Bo, etc.
Research progress in the mechanism of androgen-independent prostate cancer[J].
Chinese Journal of Andrology,2009,23(8):66-69.
[6].
Qian Subo,Shen Haibo,Cao Qifeng,et al.
Anti-androgen withdrawal Efficacy analysis of treatment of castration-resistant prostate cancer[J].
Journal of Modern Urology,2015,20(1): 40-43.
[7].
Ishikura N, Kawata H, Nishimoto A, et al.
CH5137291, an androgen receptor nuclear translocation-inhibiting compound,inhibits the growth of castration-resistant prostate cancer cells.
International Journal of Oncology.
2015;46(4):1560-1572.
[8].
Mottet N, et al.
EAU/ESTRO/ESUR/ SIOG Guidelineson Prostate Cancer 2020.
[9].
Kim N.
Chi,et al.
Apalutamide in Patients WithMetastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study.
J Clin Oncol 2021 Apr 29.
*This article is only used to provide scientific information to medical professionals, and does not represent the views of this platformCH5137291, an androgen receptor nuclear translocation-inhibiting compound,inhibits the growth of castration-resistant prostate cancer cells.
International Journal of Oncology.
2015;46(4):1560-1572.
[8].
Mottet N, et al.
EAU/ ESTRO/ESUR/SIOG Guidelineson Prostate Cancer 2020.
[9].
Kim N.
Chi,et al.
Apalutamide in Patients WithMetastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study.
J Clin Oncol 2021 Apr 29.
*This article is only used to provide scientific information to medical professionals and does not represent the views of this platformCH5137291, an androgen receptor nuclear translocation-inhibiting compound,inhibits the growth of castration-resistant prostate cancer cells.
International Journal of Oncology.
2015;46(4):1560-1572.
[8].
Mottet N, et al.
EAU/ ESTRO/ESUR/SIOG Guidelineson Prostate Cancer 2020.
[9].
Kim N.
Chi,et al.
Apalutamide in Patients WithMetastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study.
J Clin Oncol 2021 Apr 29.
*This article is only used to provide scientific information to medical professionals, and does not represent the views of this platformApalutamide in Patients WithMetastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study.
J Clin Oncol 2021 Apr 29.
*This article is only used to provide scientific information to medical professionals and does not represent the views of this platformApalutamide in Patients WithMetastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study.
J Clin Oncol 2021 Apr 29.
*This article is only used to provide scientific information to medical professionals and does not represent the views of this platform