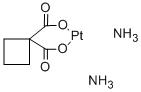
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
▎ WuXi AppTec content team editor recently, Triumvira Immunologics announced that its TAC-T cell therapy TAC01-HER2 clinical trial has completed the first patient administration for the treatment of human epidermal growth factor receptor 2 (HER2) positive solid tumors
.
Triumvira is developing innovative autologous and allogeneic T cell therapies, using the natural biology of T cells to treat patients with solid tumors and liquid tumors
.
The company's proprietary T cell antigen coupling agent (TAC) platform technology activates natural T cell functions in a different way from CAR-T and TCR-T cell therapies
.
TAC is a hybrid molecule composed of multiple protein domains.
One end of it can bind to the antigen expressed on the tumor surface.
Unlike the tumor chimeric antigen receptor (CAR), TAC does not have an intracellular co-stimulatory domain.
It binds to the T cell receptor (TCR) on the surface of T cells, and activates T cells using the natural regulatory mechanism of TCR
.
The advantage of this design is that the self-regulation mechanism of the TCR receptor is retained, so that TAC-T cells will not be activated all the time
.
Continuous activation of CAR expressed by CAR-T cells is one of the main factors leading to T cell failure
.
This design is expected to maintain the persistence of TAC-T cells and enhance their anti-cancer ability
.
▲The difference between TAC and CAR (picture source: Triumvira's official website) Compared with TCR-T cells, the binding of TAC to antigen does not need to rely on the major histocompatibility complex (MHC) to present the antigen, so it is in the target There is flexibility in the type of antigen
.
This clinical trial aims to evaluate the safety, tolerability and effectiveness of TAC01-HER2 in patients with HER2-positive metastatic, advanced, or unresectable solid tumors, and plans to recruit approximately 70 subjects
.
"Although CAR-T cell therapy has made progress in the treatment of hematological malignancies, there are still a large number of solid tumors that require safe and effective therapies
.
" said Dr.
Paul Lammers, President and CEO of Triumvira .
The launch is an important milestone for Triumvira and an opportunity to establish a clinical proof-of-concept for our TAC technology
.
"Disclaimer: WuXi AppTec's content team focuses on introducing global biomedical health research progress
.
This article is for the purpose of information exchange only.
The opinions expressed in the article do not represent the position of WuXi AppTec, nor does it mean that WuXi AppTec supports or opposes the views in the article
.
This article is not a treatment recommendation either
.
If you need guidance on the treatment plan, please go to a regular hospital for treatment
.
.
Triumvira is developing innovative autologous and allogeneic T cell therapies, using the natural biology of T cells to treat patients with solid tumors and liquid tumors
.
The company's proprietary T cell antigen coupling agent (TAC) platform technology activates natural T cell functions in a different way from CAR-T and TCR-T cell therapies
.
TAC is a hybrid molecule composed of multiple protein domains.
One end of it can bind to the antigen expressed on the tumor surface.
Unlike the tumor chimeric antigen receptor (CAR), TAC does not have an intracellular co-stimulatory domain.
It binds to the T cell receptor (TCR) on the surface of T cells, and activates T cells using the natural regulatory mechanism of TCR
.
The advantage of this design is that the self-regulation mechanism of the TCR receptor is retained, so that TAC-T cells will not be activated all the time
.
Continuous activation of CAR expressed by CAR-T cells is one of the main factors leading to T cell failure
.
This design is expected to maintain the persistence of TAC-T cells and enhance their anti-cancer ability
.
▲The difference between TAC and CAR (picture source: Triumvira's official website) Compared with TCR-T cells, the binding of TAC to antigen does not need to rely on the major histocompatibility complex (MHC) to present the antigen, so it is in the target There is flexibility in the type of antigen
.
This clinical trial aims to evaluate the safety, tolerability and effectiveness of TAC01-HER2 in patients with HER2-positive metastatic, advanced, or unresectable solid tumors, and plans to recruit approximately 70 subjects
.
"Although CAR-T cell therapy has made progress in the treatment of hematological malignancies, there are still a large number of solid tumors that require safe and effective therapies
.
" said Dr.
Paul Lammers, President and CEO of Triumvira .
The launch is an important milestone for Triumvira and an opportunity to establish a clinical proof-of-concept for our TAC technology
.
"Disclaimer: WuXi AppTec's content team focuses on introducing global biomedical health research progress
.
This article is for the purpose of information exchange only.
The opinions expressed in the article do not represent the position of WuXi AppTec, nor does it mean that WuXi AppTec supports or opposes the views in the article
.
This article is not a treatment recommendation either
.
If you need guidance on the treatment plan, please go to a regular hospital for treatment
.