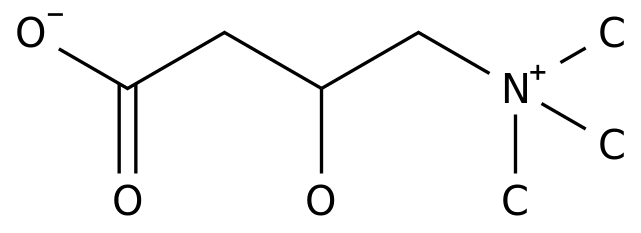
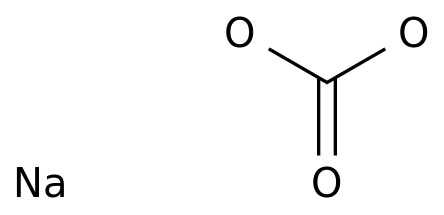
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Recently, the University of Pittsburgh School of Medicine and the UPMC research team published a presentation at Gastroenterology titled "Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations.
" that Improve the Clinical Management of Pancreatic Cysts"
.
The research team has developed a molecular assay called PancreaSeq, which enables real-time and prospectively accurate differentiation of benign cysts and probable cancerous cysts in a multi-institutional PC patient cohort, with high sensitivity and specificity
for advanced tumors.
Article published in Gastroenterology
PancreaSeq covers 22 genes
associated with pancreatic cysts.
The researchers used PancreaSeq to evaluate 97 patients who underwent EUS-FNA for pancreatic cysts and underwent follow-up diagnostic surgery (Figure 1).
The results showed that PancreaSeq detected variants
in the KRAS, GNAS and/or BRAF genes in 56 mucinous cysts.
PancreaSeq identified TP53, SMAD4, and mTOR gene variants
in 19 mucinous cysts formed by advanced tumors.
Among them, the sensitivity and specificity of MAPK/GNAS mutations for mucinous cysts are 89% and 100%, respectively, and GNAS and/or BRAF mutations are 100% specific
for IPMN.
Combined with MAPK/GNAS mutations, TP53, SMAD4, and mTOR gene mutations have 86% sensitivity and 96% specificity
for mucinous cysts formed in advanced tumors.
After excluding low-level TP53 and PIK3CA mutations, the sensitivity and specificity for advanced tumors were 86% and 100%,
respectively.
Figure 1.
Summary
of results of the retrospective PancreaSeq test.
Source: Gastroenterology
The researchers performed prospective PancreaSeq testing on 1933 ultrasound endoscopic guided fine-needle aspiration biopsy (EUS-FNA) samples, collected pancreatic cyst fluid samples from 1889 patients from 31 institutions over a two-year period, identified enough DNA for PancreaSeq testing from 1887 samples from 1832 patients, and sampled
two pancreatic cysts from 55 patients in the same EUS-FNA procedure 。 The results showed that PancreaSeq detected genetic mutations in 1,220 samples, and mutations in the KRAS, BRAF, NRAS, and HRAS genes were seen in 917, 91, 2, and 1 cysts, respectively (Figure 2).
PancreaSeq found that 158 MAPK/GNAS mutant cysts carried TP53, SMAD4, and/or mTOR gene alterations
.
In the absence of MAPK/GNAS mutations, VHL, MEN1, or two gene mutations
were observed in 125, 19, and 11 cysts, respectively.
Fig.
2.
Prospective PancreaSeq test and related imaging and pathological studies
of 1887 pancreatic cysts.
Source: Gastroenterology
The researchers obtained a total of 1,216 relevant clinicopathology data from 1,832 patients, including 1253 EUS-FNA pancreatic capsule fluid samples, of which genomic changes were detected in 851 samples and negative mutations
were detected in the remaining 402 samples.
In 159 preoperative IPMN samples, PancreaSeq detected mutations in the KRAS, BRAF, and/or GNAS genes, and in addition to MAPK/GNAS mutations, mutations in the TP53, SMAD4, and/or mTOR genes formed 77 advanced tumors, 6 MCNs advanced tumors, and 5 IPMNs with low-grade dysplasia in 90 IPMN samples (Figure 3).
Low-level point mutations in TP53, PIK3CA, PTEN, and CTNNB1 correspond to IPMNs with low dysplasia or MCNs
with low dysplasia.
Figure 3.
Representative examples
of diagnostic surgical pathology of IPNN.
Source: Gastroenterology
After the researchers ruled out 5 metastatic cancers, the sensitivity of preoperative PancreaSeq detection MAPK/GNAS mutations for mucinous cysts was 90% and specificity was 100%.
PancreaSeq has 85% sensitivity and 96% specificity
for mucinous cysts formed in advanced tumors with TP53, SMAD4 and/or mTOR gene mutations.
The sensitivity and specificity of PancreaSeq for advanced tumors with mutations in the MAPK/GNAS LOH or TP53, SMAD4, and/or mTOR genes are 87% and 99%,
respectively.
Next, the researchers evaluated the ability
of the AGA guidelines, IAP/Fukuoka guidelines, and PancreaSeq tests to identify IPMN and MCN advanced tumor formation in 246 pancreatic cysts with diagnostic pathology.
The results showed that the sensitivity and specificity of the AGA guideline for advanced tumors in mucinous cysts were 72% and 75%, respectively, and the sensitivity and specificity of the IAP/Fukuoka guideline were 84% and 52%,
respectively.
When PancreaSeq detection was added to the AGA guidelines and IAP/Fukuoka guidelines, the sensitivity increased to 96% and 98%, respectively, and the specificity remained essentially unchanged at 73% and 51%,
respectively.
Preoperative cytopathological diagnosis of endocrine tumors has 85% sensitivity and 100% specificity, and when PancreaSeq detection is combined with cytopathology, the sensitivity reaches 97% and the specificity reaches 98%.
In summary, PancreaSeq is able to accurately distinguish benign cysts from potentially cancerous cysts by sequencing pancreatic cyst-related genes and is highly specific
for advanced tumors.
PancreaSeq is more
sensitive and specific than traditional AGA guidelines and IAP/Fukuoka guidelines.
In addition, PancreaSeq is also more
accurate in distinguishing between different types of cysts than current cyst imaging-based guidelines.
Therefore, PancreaSeq can not only improve the early detection of pancreatic cancer, but also lay the foundation
for the development of prognostic biocharacteristics of pancreatic cysts.
References:
Paniccia A, Polanco PM, Boone BA, et al.
Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations that Improve the Clinical Management of Pancreatic Cysts [published online ahead of print, 2022 Oct 6].
Gastroenterology.
2022; S0016-5085(22)01086-1.
doi:10.
1053/j.
gastro.
2022.
09.
028