Hereditary angioedema (HAE) oral therapy! Biotralstat, a plasma kallikrein inhibitor of biocryst, is under review in the United States!
-
Last Update: 2020-02-19
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
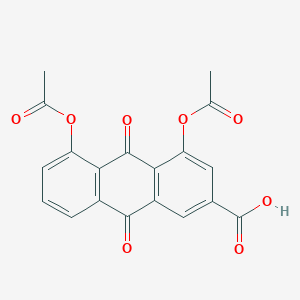
February 19, 2020 news / BIOON / -- biocryst Pharmaceutical Co., Ltd is committed to finding new, oral and small molecule drugs for the treatment of rare diseases where enzymes play a key role in the biological pathway of diseases and there are significant unmet medical needs Recently, the company announced that the U.S Food and Drug Administration (FDA) has accepted the new drug application (NDA) of bertralstat (bcx7353), a drug taken orally once a day to prevent the attack of hereditary angioedema (HAE) FDA has designated the target date of PDUFA as December 3, 2020 In the NDA acceptance letter, FDA said it does not intend to hold a meeting of the Advisory Committee to discuss NDA at present The NDA submitted this time is an oral capsule of bertralstat to prevent the attack of HAE Another oral liquid preparation of bertralstat, currently in phase II clinical, is developed for the treatment of acute hae attack The chemical structure of bertralstat (picture source: probechem CN) bertralstat is a new, oral, once a day, powerful, selective human plasma kallikrein inhibitor, currently in the late clinical development, which is used to prevent and treat the onset of angioedema in patients with hereditary angioedema (HAE) In phase III apex-2 and phase II apex-1 clinical trials, bertralstat showed good safety and tolerance In the phase III apxe-2 clinical trial, compared with placebo, bertralstat (150mg) significantly reduced the hae attack rate by 44% (P < 0.001), and in 50% of patients, the hae attack rate decreased by ≥ 70% At present, biocryst company is carrying out apex-s, which is a long-term safety clinical trial In addition, biocryst also completed the zenith-1 clinical trial, which is a phase II clinical trial of concept validation, and evaluated the treatment of acute angioedema attack with peroralstat oral liquid preparation In addition to the US FDA, biocryst plans to submit the listing application documents of biotralstat oral capsule for prevention of hae attack to Japanese drugs and medical devices (PMDA) and European Drug Administration (EMA) in the first quarter of 2020 Jon Stonehouse, CEO of biocryst, said: "the hae patient population and its doctors have been waiting for once a day oral therapy to prevent the onset of the disease The FDA accepted our berotralstat NDA and designated December as the PDUFA target date, which means that the waiting for patients and doctors is coming to an end We will build an experienced business team and carry out our business plan, and prepare to quickly launch our products to the U.S market after the approval of bertralstat " Original source: FDA accepts biocryst's NDA for oral, once daily bertralstat (bcx7353) to prevent HAE attacks
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.