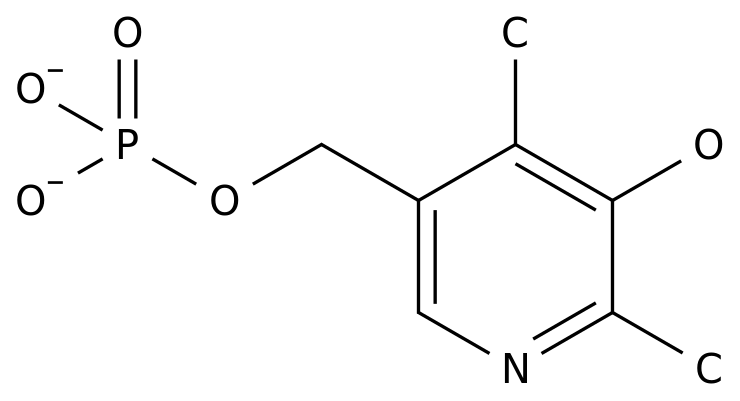
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Medical Network, April 30, reporters learned on the 29th that the innovative flu drug Sufuda (Chinese common name: Mabaloxavir) was officially approved by the China National Medical Products Administration for the treatment of acute and uncomplicated cases of 12 years old and above.
Of flu patients, including those at high risk of flu complications, will bring them more convenient treatment options.
Of flu patients, including those at high risk of flu complications, will bring them more convenient treatment options.
Influenza is a common acute respiratory infectious disease with serious consequences.
It may cause complications such as pneumonia, bronchitis, and sinusitis, and even cause death, posing a major threat to public health .
It may cause complications such as pneumonia, bronchitis, and sinusitis, and even cause death, posing a major threat to public health .
Mabaloxavir is a single-dose oral medication that can stop the virus detoxification within 24 hours, shorten the infection period and greatly reduce the duration of flu symptoms.
Clinical trials have shown that mabaloxavir is an effective treatment for previously healthy people (people without underlying diseases ) who have been infected with influenza and those at high risk of influenza complications.
Clinical trials have shown that mabaloxavir is an effective treatment for previously healthy people (people without underlying diseases ) who have been infected with influenza and those at high risk of influenza complications.
Relevant clinical trial data conducted in previously healthy, acute uncomplicated influenza patients showed that mabaloxavir is effective and well tolerated in previously healthy patients.
Compared with placebo, patients taking mabaloxavir can shorten the time of symptom improvement by 26.
5 hours, and the time of fever can be shortened by 17.
5 hours; in related clinical trials conducted in patients with high risk of influenza complications, Mabaloxa Wei has also shown effectiveness and can reduce the occurrence of complications.
Compared with placebo, the time to improve symptoms of high-risk patients taking mabaloxavir can be shortened by 29.
1 hours.
Compared with placebo, patients taking mabaloxavir can shorten the time of symptom improvement by 26.
5 hours, and the time of fever can be shortened by 17.
5 hours; in related clinical trials conducted in patients with high risk of influenza complications, Mabaloxa Wei has also shown effectiveness and can reduce the occurrence of complications.
Compared with placebo, the time to improve symptoms of high-risk patients taking mabaloxavir can be shortened by 29.
1 hours.
Zhou Hong, the president of the relevant multinational pharmaceutical company in China, said in an interview with reporters on the 29th: Under the situation that the global new crown epidemic prevention and control form is still severe, we are very happy to see that Sufuda is accelerating its approval in China.
We hope that this drug with a brand-new mechanism of action can effectively help the prevention and treatment of potential large-scale influenza in the winter and spring seasons with high influenza incidence, and provide an important supplement to existing clinical influenza treatment programs.
"(Finish)
We hope that this drug with a brand-new mechanism of action can effectively help the prevention and treatment of potential large-scale influenza in the winter and spring seasons with high influenza incidence, and provide an important supplement to existing clinical influenza treatment programs.
"(Finish)
Medical Network, April 30, reporters learned on the 29th that the innovative flu drug Sufuda (Chinese common name: Mabaloxavir) was officially approved by the China National Medical Products Administration for the treatment of acute and uncomplicated cases of 12 years old and above.
Of flu patients, including those at high risk of flu complications, will bring them more convenient treatment options.
Of flu patients, including those at high risk of flu complications, will bring them more convenient treatment options.
Influenza is a common acute respiratory infectious disease with serious consequences.
It may cause complications such as pneumonia, bronchitis, and sinusitis, and even cause death, posing a major threat to public health .
It may cause complications such as pneumonia, bronchitis, and sinusitis, and even cause death, posing a major threat to public health .
Mabaloxavir is a single-dose oral medication that can stop the virus detoxification within 24 hours, shorten the infection period and greatly reduce the duration of flu symptoms.
Clinical trials have shown that mabaloxavir is an effective treatment for previously healthy people (people without underlying diseases ) who have been infected with influenza and those at high risk of influenza complications.
Clinical trials have shown that mabaloxavir is an effective treatment for previously healthy people (people without underlying diseases ) who have been infected with influenza and those at high risk of influenza complications.
Relevant clinical trial data conducted in previously healthy, acute uncomplicated influenza patients showed that mabaloxavir is effective and well tolerated in previously healthy patients.
Compared with placebo, patients taking mabaloxavir can shorten the time of symptom improvement by 26.
5 hours, and the time of fever can be shortened by 17.
5 hours; in related clinical trials conducted in patients with high risk of influenza complications, Mabaloxa Wei has also shown effectiveness and can reduce the occurrence of complications.
Compared with placebo, the time to improve symptoms of high-risk patients taking mabaloxavir can be shortened by 29.
1 hours.
Compared with placebo, patients taking mabaloxavir can shorten the time of symptom improvement by 26.
5 hours, and the time of fever can be shortened by 17.
5 hours; in related clinical trials conducted in patients with high risk of influenza complications, Mabaloxa Wei has also shown effectiveness and can reduce the occurrence of complications.
Compared with placebo, the time to improve symptoms of high-risk patients taking mabaloxavir can be shortened by 29.
1 hours.
Zhou Hong, the president of the relevant multinational pharmaceutical company in China, said in an interview with reporters on the 29th: Under the situation that the global new crown epidemic prevention and control form is still severe, we are very happy to see that Sufuda is accelerating its approval in China.
We hope that this drug with a brand-new mechanism of action can effectively help the prevention and treatment of potential large-scale influenza in the winter and spring seasons with high influenza incidence, and provide an important supplement to existing clinical influenza treatment programs.
"(Finish)
We hope that this drug with a brand-new mechanism of action can effectively help the prevention and treatment of potential large-scale influenza in the winter and spring seasons with high influenza incidence, and provide an important supplement to existing clinical influenza treatment programs.
"(Finish)
Medical Network, April 30, reporters learned on the 29th that the innovative flu drug Sufuda (Chinese common name: Mabaloxavir) was officially approved by the China National Medical Products Administration for the treatment of acute and uncomplicated cases of 12 years old and above.
Of flu patients, including those at high risk of flu complications, will bring them more convenient treatment options.
Of flu patients, including those at high risk of flu complications, will bring them more convenient treatment options.
Influenza is a common acute respiratory infectious disease with serious consequences.
It may cause complications such as pneumonia, bronchitis, and sinusitis, and even cause death, posing a major threat to public health .
Healthy, healthy, healthyIt may cause complications such as pneumonia, bronchitis, and sinusitis, and even cause death, posing a major threat to public health .
Mabaloxavir is a single-dose oral medication that can stop the virus detoxification within 24 hours, shorten the infection period and greatly reduce the duration of flu symptoms.
Clinical trials have shown that mabaloxavir is an effective treatment for previously healthy people (people without underlying diseases ) who have been infected with influenza and those at high risk of influenza complications.
Disease disease diseaseClinical trials have shown that mabaloxavir is an effective treatment for previously healthy people (people without underlying diseases ) who have been infected with influenza and those at high risk of influenza complications.
Relevant clinical trial data conducted in previously healthy, acute uncomplicated influenza patients showed that mabaloxavir is effective and well tolerated in previously healthy patients.
Compared with placebo, patients taking mabaloxavir can shorten the time of symptom improvement by 26.
5 hours, and the time of fever can be shortened by 17.
5 hours; in related clinical trials conducted in patients with high risk of influenza complications, Mabaloxa Wei has also shown effectiveness and can reduce the occurrence of complications.
Compared with placebo, the time to improve symptoms of high-risk patients taking mabaloxavir can be shortened by 29.
1 hours.
Compared with placebo, patients taking mabaloxavir can shorten the time of symptom improvement by 26.
5 hours, and the time of fever can be shortened by 17.
5 hours; in related clinical trials conducted in patients with high risk of influenza complications, Mabaloxa Wei has also shown effectiveness and can reduce the occurrence of complications.
Compared with placebo, the time to improve symptoms of high-risk patients taking mabaloxavir can be shortened by 29.
1 hours.
Zhou Hong, the president of the relevant multinational pharmaceutical company in China, said in an interview with reporters on the 29th: Under the situation that the global new crown epidemic prevention and control form is still severe, we are very happy to see that Sufuda is accelerating its approval in China.
We hope that this drug with a brand-new mechanism of action can effectively help the prevention and treatment of potential large-scale influenza in the winter and spring seasons with high influenza incidence, and provide an important supplement to existing clinical influenza treatment programs.
"(Finish)
We hope that this drug with a brand-new mechanism of action can effectively help the prevention and treatment of potential large-scale influenza in the winter and spring seasons with high influenza incidence, and provide an important supplement to existing clinical influenza treatment programs.
"(Finish)