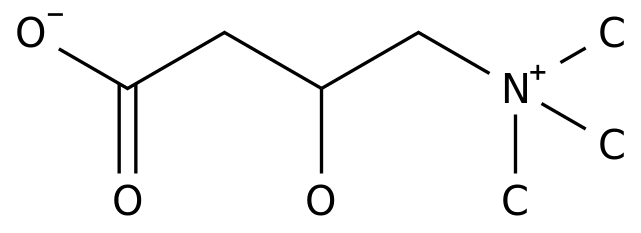
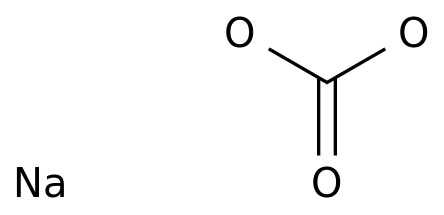
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Currently, most cancer diagnoses require surgical incisional tissue biopsies or sample analysis from suspicious tumor sites
.
These invasive methods are time-consuming, costly,
and traumatic.
This article is the original of Translational Medicine Network, please indicate the source for reprinting
Written by Jevin
Artificial intelligence identifies the microbiota in the blood
01
In 2020, a study published in Nature by the University of California, San Diego, showed that by training artificial intelligence to identify the characteristics of the microbiota in the blood, not only can cancer be identified, but even different types of cancer can be distinguished
.
This microbiome-based oncology diagnostic tool promises to change the way
people screen and diagnose cancer.
Researchers use simple blood or microbial DNA tests to determine whether cancer is present and to distinguish the type of
cancer.
The analysis results show that the machine learning model can not only distinguish between cancerous and cancer-free samples, but also distinguish different types of tumors: the sensitivity of identifying lung cancer patients is 86%, the specificity of identifying people without lung disease is as high as 100%, and the accuracy of distinguishing between prostate cancer and lung cancer is 81%.
Artificial intelligence detects changes in DNA fragments of cancer cells in the blood
02
In 2021, researchers at Hopkins University developed a new artificial intelligence blood test technology to detect lung cancer in a new study of 724 people, detecting more than 80% of liver cancers
.
Their findings, published Nov.
18 in Cancer Research, aim to precisely prevent, detect and intercept cancer
.
This blood test is called DELFI (Early Intercepted DNA Fragment Assessment) and detects changes
in cancer cells' DNA fragments flowing into the bloodstream.
The researchers used DELFI technology to test plasma samples taken from 724 individuals in the United States, the European Union and Hong Kong, China, to train and validate machine learning models and detect hepatocellular carcinoma (HCC).
Sensitivity and specificity of novel tests
03
DELFI technology uses blood tests to measure how DNA is assembled within the nucleus by studying the size and amount
of cell-free DNA (cfDNA) from different regions of the genome in circulation.
In contrast, when cancer cells die, they release pieces of DNA into the bloodstream
in a chaotic way.
In the latest study, the researchers tested cfDNA fragments isolated from plasma samples, a test previously shown to accurately classify
lung cancer.
They analyzed the fragmentation patterns of each sample to arrive at the DELFI score
.
Among them, non-cancer patients with viral hepatitis or cirrhosis scored lower, but in the US/EU sample, 75 liver cancer patients scored an average of 5 to 10 times higher, with higher scores observed in all cancer stages, including early-stage disease
.
In addition, the test detected fragmented changes in the content and packaging of the liver cancer genome, including genomic regions
associated with liver-specific activity.
DELFI technology detects liver cancer at an early stage with an overall sensitivity or ability to accurately detect cancer with an overall sensitivity or ability to accurately detect cancer with a specificity of 98%.
In samples collected from people at high risk of liver cancer, the test had 85% sensitivity and 80% specificity
.
The researchers added: "Currently, less than 20% of high-risk populations are screened
for liver cancer due to accessibility and suboptimal test performance.
This new blood test could double the number of liver cancer cases detected compared to the standard blood tests available
.
”
Resources:
http://stdaily.
com/index/kejixinwen/202211/6449168cf5264638af1261f8cd9bdb1a.
shtml
Note: This article is intended to introduce the progress of medical research and cannot be used as a reference
for treatment options.
If you need health guidance, please go to a regular hospital
.
Forum One
Tumor immunity "Pujiang Discussion" schedule
Forum II
Tumor immunity "Matsue traceability" schedule
Forum III
Oncology drug development and treatment schedule
Forum V
Oncology frontier "pinnacle theory" agenda
Forum VI
Breast Cancer Frontier and Clinical Translational Agenda
(Click above to view the detailed schedule)