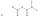-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read and refer to the top issue of the new article, Prof.
Lin Yansong and you detail the REALITY research
.
Differentiated thyroid cancer (DTC) accounts for more than 94% of thyroid cancers.
Some intermediate and high-risk DTC patients, especially metastatic DTC patients, will still progress to radioactive iodine-refractory DTC after treatment.
(RAIR-DTC), the 10-year survival rate is less than 10%
.
In addition, the prognosis of thyroid cancer in China is 14% different from that in developed countries such as the United States (84.
3% vs 98.
3%), which indirectly suggests that there are far more RAIR-DTC patients threatening the survival of patients in China than in other countries
.
On December 16, 2021, JAMA Oncology, a top journal in oncology, published online the results of the Phase III REALITY study of "apatinib or placebo in locally advanced or metastatic RAIR-DTC"
.
The "Medical Tumor Channel" specially invited the first author of the study, Professor Lin Yansong of Peking Union Medical College Hospital, to conduct an in-depth interpretation of the study, so as to deliver the latest academic essence to the majority of doctors and patients
.
Behind the gratifying efficacy data is a team's years of "deep cultivation" in the medical field: What is the original intention of you and your team to carry out the REALITY study? ▎Professor Lin Yansong: RAIR-DTC is an international difficulty in the field of thyroid, and it is also the focus of our multidisciplinary team of Peking Union Medical College Hospital since 2011
.
As early as 2012, the team in our hospital took the lead in using integrin receptor imaging to locate and reveal the characteristics of high expression of integrin receptors and rich blood supply in RAIR-DTC lesions, which is also the basis for the subsequent use of anti-angiogenic drugs.
After treatment of RAIR-DTC provided molecular imaging evidence, we then explored the relationship between the genetic signature of thyroid cancer primary foci and the iodine uptake signature of distant metastases, as well as the use of semiquantitative changes in serum markers of thyroid cancer to predict RAIR trends, these studies allow us to judge and predict RAIR-DTC earlier from multiple dimensions
.
With the in-depth understanding of the molecular biology of thyroid cancer, people gradually realize that as a tumor with a rich blood supply, drugs targeting the vascular endothelial growth factor (VEGF-VEGFR) signaling pathway may become the treatment option for RAIR-DTC
.
In 2013 and 2015, the U.
S.
Food and Drug Administration (FDA) approved the indications of sorafenib and lenvatinib for the treatment of RAIR-DTC based on the results of the DECISION and SELECT studies, respectively.
At the end of the year, it was approved in China one after another
.
Prior to this, there was a huge unmet therapeutic need for RAIR-DTC patients in China
.
Actively seeking accessible and effective treatments for these incurable RAIR-DTC patients is the original intention of our team to carry out relevant research
.
In 2016, our hospital officially launched a small-sample, exploratory phase II study of apatinib in the treatment of RAIR-DTC, and has since reported the short-term, medium-term, long-term and different dose groups (750mg) of apatinib to the international community.
/d and 500mg/d) distinct efficacy and safety
.
And reported the final results of the phase II study in 2020 (median follow-up 42 months), the apatinib treatment group showed a very good progression-free survival benefit, with a median progression-free survival (mPFS) of more than 18 month
.
Our hospital has published a number of SCI articles around the Phase II series of studies, reflecting the academic attitude of Peking Union Medical College Hospital of "rigorous refinement" and laying a solid foundation for the follow-up Phase III studies
.
To meet the needs of patients, the survival data of apatinib in the treatment of RAIR-DTC is impressive.
Q Medical community: What are the results of the REALITY study? ▎Professor Lin Yansong: On the basis of the Phase II exploratory study, this REALITY Phase III study strictly limited the enrollment conditions, and the baseline level of the patients was very uniform
.
A total of 92 patients with progressive locally advanced or metastatic RAIR-DTC were enrolled between February 2017 and March 2020
.
RESULTS: mPFS was 22.
2 months (95% CI 10.
91-NE) in the apatinib arm and 4.
5 months (95% CI 1.
94-9.
17) in the placebo arm (HR, 0.
26; 95% CI 0.
14-0.
47) ; P<0.
001)
.
The objective response rate (ORR) was 54.
3% (95% CI 39.
0%-69.
1%) and the disease control rate (DCR) was 95.
7% (95% CI 85.
2%-99.
5%) in the apatinib group compared with placebo.
were 2.
2% (95% CI 0.
1%-11.
5%) and 58.
7% (95% CI 43.
2%-73.
0%), respectively
.
It is remarkable that, unlike the DECISION and SELECT studies, which did not observe an overall survival (OS) benefit, patients in the apatinib group in the REALITY study achieved a significant OS benefit, and the apatinib group achieved a significant OS benefit.
Median OS was not reached (95% CI, 26.
25-NE) and 29.
9 months (95% CI 18.
96-NE) in the and placebo groups, respectively (HR 0.
42; 95% CI 0.
18-0.
97; P=0.
04) It was suggested that apatinib reduced the risk of death by 58%
.
"Local research" has established the status of anti-angiogenesis therapy, and follow-up research needs to "keep up" Q medical community: What is the significance of REALITY research to clinical practice in my country? What room for improvement is there? ▎Professor Lin Yansong: As a study designed by Chinese scholars and implemented in a large sample population in China, the REALITY study verifies the efficacy of the original Chinese drug apatinib on RAIR-DTC patients, which is the significance of the REALITY study
.
At the same time, the results of the REALITY study further demonstrated the status of anti-angiogenic drugs in the first-line treatment of RAIR-DTC, laying the foundation for subsequent studies
.
The results of the REALITY study were also announced in the form of a speech at the 2020 European Society for Medical Oncology (ESMO) annual meeting, and were selected for the 2020 ESMO Highlights, and finally published online in full text in JAMA Oncology in December 2021, which reflects the international colleagues Recognition of the research foundation, research design, implementation quality, research results and clinical translational significance of the REALITY study
.
The REALITY study has brought an effective, accessible and relatively inexpensive treatment option for Chinese RAIR-DTC patients.
We also hope to continue to explore apatinib in the follow-up study and expand the research scale to the international level
.
Of course, the REALITY study also has certain limitations, namely, its placebo-controlled research design.
This is because there are still no drugs available for such patients in China during the study period, so the use of placebo control is unavoidable.
We We also look forward to future head-to-head studies to further validate the efficacy of apatinib
.
The problem of drug resistance still needs to be overcome.
Combination therapy is the key.
Q The medical community: What are the directions to be explored in the future for the treatment of RAIR-DTC? ▎Professor Lin Yansong: Unlike lung cancer and other "large tumors", the molecular level exploration of thyroid cancer has just started, its oncogenic driver genes have not been identified, and molecular markers predicting the efficacy of apatinib have not yet been discovered
.
Therefore, looking for molecular markers to predict the efficacy and resistance mechanisms of apatinib in the later analysis of the study is one of the future directions of REALITY research
.
A number of current phase III studies have confirmed the role of anti-angiogenic drugs in the first-line treatment of RAIR-DTC, but the accompanying drug resistance phenomenon still needs to be overcome
.
The patients enrolled in the REALITY study included patients who were resistant to first-line treatment of sorafenib and lenvatinib.
The effectiveness of apatinib as a second-line treatment has been proven, but apatinib also has resistance problems.
Therefore, researchers are also exploring treatment strategies after apatinib resistance, such as anti-angiogenic drugs combined with immunotherapy, anti-angiogenic drugs combined with radioactive iodine therapy,
etc.
In view of the fact that the combination of apatinib and camrelizumab has obtained certain clinical evidence in lung cancer, liver cancer, esophageal cancer and other tumor types, the Peking Union Medical College Hospital is currently carrying out "double AI" therapy for anti-vascular disease.
The phase II study of generating drug-resistant patients is over halfway through.
Whether anti-angiogenesis + immunotherapy will achieve "1+1>2" effect is worth looking forward to
.
Acknowledgments Finally, Prof.
Lin Yansong expressed his special thanks to Prof.
Qin Shukui from Nanjing Jinling Hospital for his guidance and strong support for this study, and also to Prof.
Li Zhiyong from the Affiliated Hospital of Xuzhou Medical University, Prof.
Yang Hui from Henan Cancer Hospital, Prof.
Fu Wei from the Affiliated Hospital of Guilin Medical College, and Zhongshan Professor Li Shaohua from the Cancer Hospital Affiliated to the University, Professor Chen Wenxin from the Fujian Provincial Hospital, Professor Gao Zairong from the Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Professor Miao Weibing from the First Affiliated Hospital of Fujian Medical University, Professor Xu Huiqin from the First Affiliated Hospital of Anhui Medical University, and No.
Professor Zhang Qing from Affiliated Hospital, Professor Zhao Xinming from the Fourth Hospital of Hebei Medical University, Professor Bao Jiandong from Jiangyuan Hospital of Jiangsu Province, Professor Li Linfa from Zhejiang Cancer Hospital, Professor Ren Yuan from Shanxi Cancer Hospital, Professor Lin Chenghe from the First Hospital of Jilin University, Hebei Medical University Professor Jing Shanghua of the Fourth Hospital, Professor Ma Qingjie of the Sino-Japanese Friendship Hospital of Jilin University, Professor Liang Jun of Peking University International Hospital, Professor Chen Guang of the First Hospital of Jilin University, Professor Zhang Hong of Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Ruijin Affiliated to Shanghai Jiaotong University School of Medicine Professor Zhang Yifan of the hospital and other experts from the participating centers for their rigorous academic attitude and full cooperation; thanks to other researchers in the participating centers for their hard work; thanks to all the subjects and their families for their trust and support in us; finally, thanks to Hengrui Medicine for the REALITY research full support
.
Expert Profile Prof.
Lin Yansong, Ph.
D.
in Nuclear Medicine, Postdoctoral Fellow, Chief Physician, Peking Union Medical College Hospital, Professor, Chinese Academy of Medical Sciences, Nuclear Medicine, Ph.
D.
Supervisor Chairman of the Expert Committee Chairman of the Chinese Medical Doctor Association Thyroid Science Expert Committee Chairman of the Beijing Nuclear Medicine Branch Therapeutic Group Leader Executive editorial board member of the Journal of Nuclear Medicine and Molecular Imaging "Chinese Journal of Medicine" (English version) editorial board member of the Ministry of Health Professional and Technical Title Examination Expert Committee Final Review Expert International Atomic Energy Agency IAEA RAS6043 Project Coordinator in China Reference: [1]Lin Y , et al.
JAMA Oncol.
2021 Dec 16.
doi: 10.
1001/jamaoncol.
2021.
6268.
https://jamanetwork.
com/journals/jamaoncology/fullarticle/2786815
Lin Yansong and you detail the REALITY research
.
Differentiated thyroid cancer (DTC) accounts for more than 94% of thyroid cancers.
Some intermediate and high-risk DTC patients, especially metastatic DTC patients, will still progress to radioactive iodine-refractory DTC after treatment.
(RAIR-DTC), the 10-year survival rate is less than 10%
.
In addition, the prognosis of thyroid cancer in China is 14% different from that in developed countries such as the United States (84.
3% vs 98.
3%), which indirectly suggests that there are far more RAIR-DTC patients threatening the survival of patients in China than in other countries
.
On December 16, 2021, JAMA Oncology, a top journal in oncology, published online the results of the Phase III REALITY study of "apatinib or placebo in locally advanced or metastatic RAIR-DTC"
.
The "Medical Tumor Channel" specially invited the first author of the study, Professor Lin Yansong of Peking Union Medical College Hospital, to conduct an in-depth interpretation of the study, so as to deliver the latest academic essence to the majority of doctors and patients
.
Behind the gratifying efficacy data is a team's years of "deep cultivation" in the medical field: What is the original intention of you and your team to carry out the REALITY study? ▎Professor Lin Yansong: RAIR-DTC is an international difficulty in the field of thyroid, and it is also the focus of our multidisciplinary team of Peking Union Medical College Hospital since 2011
.
As early as 2012, the team in our hospital took the lead in using integrin receptor imaging to locate and reveal the characteristics of high expression of integrin receptors and rich blood supply in RAIR-DTC lesions, which is also the basis for the subsequent use of anti-angiogenic drugs.
After treatment of RAIR-DTC provided molecular imaging evidence, we then explored the relationship between the genetic signature of thyroid cancer primary foci and the iodine uptake signature of distant metastases, as well as the use of semiquantitative changes in serum markers of thyroid cancer to predict RAIR trends, these studies allow us to judge and predict RAIR-DTC earlier from multiple dimensions
.
With the in-depth understanding of the molecular biology of thyroid cancer, people gradually realize that as a tumor with a rich blood supply, drugs targeting the vascular endothelial growth factor (VEGF-VEGFR) signaling pathway may become the treatment option for RAIR-DTC
.
In 2013 and 2015, the U.
S.
Food and Drug Administration (FDA) approved the indications of sorafenib and lenvatinib for the treatment of RAIR-DTC based on the results of the DECISION and SELECT studies, respectively.
At the end of the year, it was approved in China one after another
.
Prior to this, there was a huge unmet therapeutic need for RAIR-DTC patients in China
.
Actively seeking accessible and effective treatments for these incurable RAIR-DTC patients is the original intention of our team to carry out relevant research
.
In 2016, our hospital officially launched a small-sample, exploratory phase II study of apatinib in the treatment of RAIR-DTC, and has since reported the short-term, medium-term, long-term and different dose groups (750mg) of apatinib to the international community.
/d and 500mg/d) distinct efficacy and safety
.
And reported the final results of the phase II study in 2020 (median follow-up 42 months), the apatinib treatment group showed a very good progression-free survival benefit, with a median progression-free survival (mPFS) of more than 18 month
.
Our hospital has published a number of SCI articles around the Phase II series of studies, reflecting the academic attitude of Peking Union Medical College Hospital of "rigorous refinement" and laying a solid foundation for the follow-up Phase III studies
.
To meet the needs of patients, the survival data of apatinib in the treatment of RAIR-DTC is impressive.
Q Medical community: What are the results of the REALITY study? ▎Professor Lin Yansong: On the basis of the Phase II exploratory study, this REALITY Phase III study strictly limited the enrollment conditions, and the baseline level of the patients was very uniform
.
A total of 92 patients with progressive locally advanced or metastatic RAIR-DTC were enrolled between February 2017 and March 2020
.
RESULTS: mPFS was 22.
2 months (95% CI 10.
91-NE) in the apatinib arm and 4.
5 months (95% CI 1.
94-9.
17) in the placebo arm (HR, 0.
26; 95% CI 0.
14-0.
47) ; P<0.
001)
.
The objective response rate (ORR) was 54.
3% (95% CI 39.
0%-69.
1%) and the disease control rate (DCR) was 95.
7% (95% CI 85.
2%-99.
5%) in the apatinib group compared with placebo.
were 2.
2% (95% CI 0.
1%-11.
5%) and 58.
7% (95% CI 43.
2%-73.
0%), respectively
.
It is remarkable that, unlike the DECISION and SELECT studies, which did not observe an overall survival (OS) benefit, patients in the apatinib group in the REALITY study achieved a significant OS benefit, and the apatinib group achieved a significant OS benefit.
Median OS was not reached (95% CI, 26.
25-NE) and 29.
9 months (95% CI 18.
96-NE) in the and placebo groups, respectively (HR 0.
42; 95% CI 0.
18-0.
97; P=0.
04) It was suggested that apatinib reduced the risk of death by 58%
.
"Local research" has established the status of anti-angiogenesis therapy, and follow-up research needs to "keep up" Q medical community: What is the significance of REALITY research to clinical practice in my country? What room for improvement is there? ▎Professor Lin Yansong: As a study designed by Chinese scholars and implemented in a large sample population in China, the REALITY study verifies the efficacy of the original Chinese drug apatinib on RAIR-DTC patients, which is the significance of the REALITY study
.
At the same time, the results of the REALITY study further demonstrated the status of anti-angiogenic drugs in the first-line treatment of RAIR-DTC, laying the foundation for subsequent studies
.
The results of the REALITY study were also announced in the form of a speech at the 2020 European Society for Medical Oncology (ESMO) annual meeting, and were selected for the 2020 ESMO Highlights, and finally published online in full text in JAMA Oncology in December 2021, which reflects the international colleagues Recognition of the research foundation, research design, implementation quality, research results and clinical translational significance of the REALITY study
.
The REALITY study has brought an effective, accessible and relatively inexpensive treatment option for Chinese RAIR-DTC patients.
We also hope to continue to explore apatinib in the follow-up study and expand the research scale to the international level
.
Of course, the REALITY study also has certain limitations, namely, its placebo-controlled research design.
This is because there are still no drugs available for such patients in China during the study period, so the use of placebo control is unavoidable.
We We also look forward to future head-to-head studies to further validate the efficacy of apatinib
.
The problem of drug resistance still needs to be overcome.
Combination therapy is the key.
Q The medical community: What are the directions to be explored in the future for the treatment of RAIR-DTC? ▎Professor Lin Yansong: Unlike lung cancer and other "large tumors", the molecular level exploration of thyroid cancer has just started, its oncogenic driver genes have not been identified, and molecular markers predicting the efficacy of apatinib have not yet been discovered
.
Therefore, looking for molecular markers to predict the efficacy and resistance mechanisms of apatinib in the later analysis of the study is one of the future directions of REALITY research
.
A number of current phase III studies have confirmed the role of anti-angiogenic drugs in the first-line treatment of RAIR-DTC, but the accompanying drug resistance phenomenon still needs to be overcome
.
The patients enrolled in the REALITY study included patients who were resistant to first-line treatment of sorafenib and lenvatinib.
The effectiveness of apatinib as a second-line treatment has been proven, but apatinib also has resistance problems.
Therefore, researchers are also exploring treatment strategies after apatinib resistance, such as anti-angiogenic drugs combined with immunotherapy, anti-angiogenic drugs combined with radioactive iodine therapy,
etc.
In view of the fact that the combination of apatinib and camrelizumab has obtained certain clinical evidence in lung cancer, liver cancer, esophageal cancer and other tumor types, the Peking Union Medical College Hospital is currently carrying out "double AI" therapy for anti-vascular disease.
The phase II study of generating drug-resistant patients is over halfway through.
Whether anti-angiogenesis + immunotherapy will achieve "1+1>2" effect is worth looking forward to
.
Acknowledgments Finally, Prof.
Lin Yansong expressed his special thanks to Prof.
Qin Shukui from Nanjing Jinling Hospital for his guidance and strong support for this study, and also to Prof.
Li Zhiyong from the Affiliated Hospital of Xuzhou Medical University, Prof.
Yang Hui from Henan Cancer Hospital, Prof.
Fu Wei from the Affiliated Hospital of Guilin Medical College, and Zhongshan Professor Li Shaohua from the Cancer Hospital Affiliated to the University, Professor Chen Wenxin from the Fujian Provincial Hospital, Professor Gao Zairong from the Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Professor Miao Weibing from the First Affiliated Hospital of Fujian Medical University, Professor Xu Huiqin from the First Affiliated Hospital of Anhui Medical University, and No.
Professor Zhang Qing from Affiliated Hospital, Professor Zhao Xinming from the Fourth Hospital of Hebei Medical University, Professor Bao Jiandong from Jiangyuan Hospital of Jiangsu Province, Professor Li Linfa from Zhejiang Cancer Hospital, Professor Ren Yuan from Shanxi Cancer Hospital, Professor Lin Chenghe from the First Hospital of Jilin University, Hebei Medical University Professor Jing Shanghua of the Fourth Hospital, Professor Ma Qingjie of the Sino-Japanese Friendship Hospital of Jilin University, Professor Liang Jun of Peking University International Hospital, Professor Chen Guang of the First Hospital of Jilin University, Professor Zhang Hong of Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Ruijin Affiliated to Shanghai Jiaotong University School of Medicine Professor Zhang Yifan of the hospital and other experts from the participating centers for their rigorous academic attitude and full cooperation; thanks to other researchers in the participating centers for their hard work; thanks to all the subjects and their families for their trust and support in us; finally, thanks to Hengrui Medicine for the REALITY research full support
.
Expert Profile Prof.
Lin Yansong, Ph.
D.
in Nuclear Medicine, Postdoctoral Fellow, Chief Physician, Peking Union Medical College Hospital, Professor, Chinese Academy of Medical Sciences, Nuclear Medicine, Ph.
D.
Supervisor Chairman of the Expert Committee Chairman of the Chinese Medical Doctor Association Thyroid Science Expert Committee Chairman of the Beijing Nuclear Medicine Branch Therapeutic Group Leader Executive editorial board member of the Journal of Nuclear Medicine and Molecular Imaging "Chinese Journal of Medicine" (English version) editorial board member of the Ministry of Health Professional and Technical Title Examination Expert Committee Final Review Expert International Atomic Energy Agency IAEA RAS6043 Project Coordinator in China Reference: [1]Lin Y , et al.
JAMA Oncol.
2021 Dec 16.
doi: 10.
1001/jamaoncol.
2021.
6268.
https://jamanetwork.
com/journals/jamaoncology/fullarticle/2786815