-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
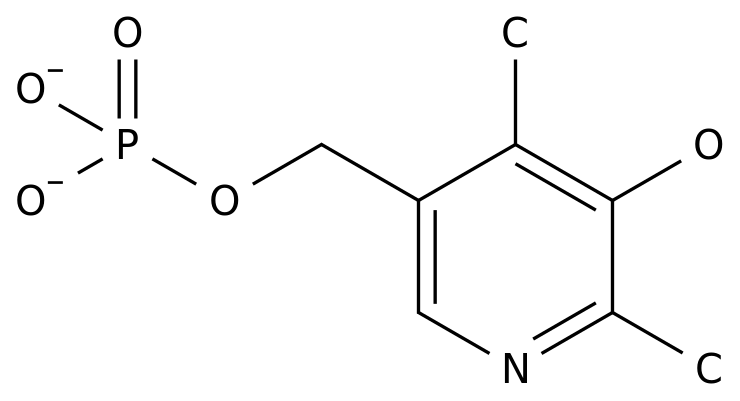
Mb07133, a new liver cancer targeting drug developed by Xi'an Xintong Pharmaceutical Research Co., Ltd., has recently been approved by the State Food and Drug Administration for clinical research, which marks a major breakthrough in the research of innovative drugs for liver cancer in China Mb07133 is a new targeted drug for the treatment of advanced liver cancer Based on the liver targeting technology, mb07133 can concentrate the drug concentration in the liver, improve the curative effect, significantly reduce the systemic exposure of the drug, and reduce the toxicity of plasma, bone marrow and extrahepatic tissues
Li Xiuzhen, deputy general manager of Xi'an Xintong Pharmaceutical Research Co., Ltd.: when our drug arrives in the body, it will not release the drug when it does not hit the liver during the whole operation process Only when it arrives at the liver, there is a special enzyme in the liver that can release it, like a key to open it and act on it If it moves to other places, it doesn't have this enzyme, it can't release it, and it will not damage other places Prior to that, another new hepatitis B targeting drug, paradofovir, was approved for clinical study on October 17, 2013 Mb07133 is the prodrug of cytarabine, and Xintong company uses liver targeting technology to give cytarabine liver targeting, which can be targeted into the liver The concentration of cytarabine in the liver is 15 times of that in the blood and 10 times of that in the bone marrow; the concentration in the liver is 250 times of that in the blood after 7 days of intravenous administration It significantly reduced the systemic exposure of cytarabine, and reduced the toxicity of plasma, bone marrow and extrahepatic tissues In China, there are 51000 new cases of liver cancer every year, accounting for 53.6% of the total number of new cases in the world Nearly 425000 people die of liver cancer every year According to the clinical guidelines for HCC issued by the American Society of Hepatology research, the systemic application of chemotherapeutics has no definite effect, but the effect of local perfusion of chemotherapeutics via hepatic artery is quite positive Cytosine arabinoside is a classic anti metabolism drug, which is widely used in cytotoxic anticancer drugs It can inhibit DNA synthesis by inhibiting DNA polymerase and * * cell proliferation It is mostly used in hepatic artery intubation chemotherapy and leukemia chemotherapy Mb07133 is based on liver targeting technology, which makes cytarabine concentrate in the liver, avoids hepatic artery intubation, improves the curative effect and reduces the toxicity of extrahepatic tissues The toxicity test showed that mb07133 was well tolerated to mice, rats and dogs, and no obvious toxicity was found during the administration Mb07133 had no hepatotoxicity in rats and dogs after 7 days of intravenous injection and 21 days of recovery The results of phase I clinical trials in the United States, Hong Kong and Taiwan showed that the average survival time of mb07133 for patients with advanced liver cancer was 9.7 months, of which 30% patients had a longer survival time, up to 14.9 months, while the sorafenib produced by Bayer, the leading drug for the treatment of liver cancer, was 6.5 months Sorafenib costs 25192 yuan per box and 1664 yuan per day It is estimated that after mb07133 is put on the market, the daily cost of medicine will be 500 yuan, which will greatly reduce the economic burden of patients while satisfying the clinical drug use of patients According to reports, at present, the only effective drug approved by FDA for liver cancer in the world is sorafenib toluene sulfonate (dogimer) from Bayer company in Germany The survival period of patients is 6.5 months, the price of each box is up to 25192 yuan, and the daily cost of medication is 1664 yuan Based on liver targeting technology, mb07133, a class 1.1 new drug, was developed by Xintong company to target advanced liver cancer The concentration of mb07133 in liver was 15 times of that in blood and 10 times of that in bone marrow After intravenous administration for 7 days, the concentration in liver was 250 times of that in blood Besides avoiding hepatic artery intubation, improving the therapeutic effect, the systemic exposure of drugs was greatly reduced, and the reduction was significant The toxicity of plasma, bone marrow and extrahepatic tissue was studied Secondly, in foreign clinical trials, the average survival time of patients was 9.7 months, 29% of patients had a longer survival time, up to 14.9 months, and its efficacy and safety were superior to sorafenib Thirdly, after mb07133 was put on the market, the daily drug cost was expected to be 500 yuan, which would greatly reduce the patient's experience while satisfying the patients' clinical drug use The financial burden has brought good news to the majority of patients with liver cancer, and it is expected to become the first-line treatment drug of choice for patients with liver cancer