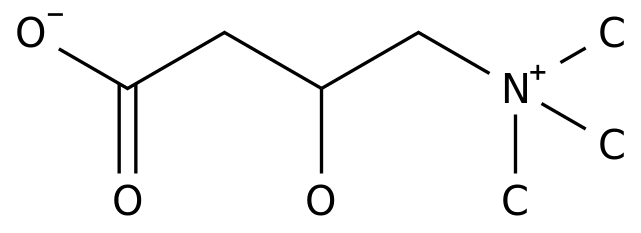
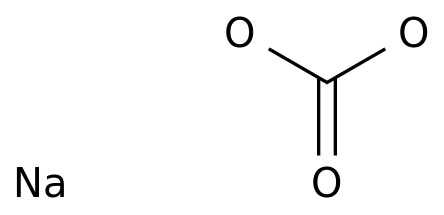
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Recent popular reports from Yimaike★Tomorrow! Nanjing JiangbeiThe annual event in the field of nucleic acid drugs is about to open ★One of the most popular research outlets! Nucleic acid cooperation investment transaction inventoryMedClub New Observation May 7, 2021/MedClub News/- May 5, Merck & Co.
(MSD) announced that the US Food and Drug Administration (FDA) approved its drug PD-1 therapy Keytruda, trastuzumab, fluoropyrimidine, and platinum-based chemotherapy are used in the first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric cancer or gastroesophageal junction (GEJ) adenocarcinoma patients .
Dr.
Roy Baynes, MSD Research Laboratories Senior Vice President and Chief Medical Officer, Chief Medical Officer, said: "This is the first time the FDA has approved PD-1 therapy in combination with trastuzumab and chemotherapy as the first-line treatment for such patients.
We are very pleased with KEYTRUDA's new first-line combination treatment plan.
Compared with the standard treatment for patients with HER2-positive gastric cancer and GEJ cancer, Keytruda combination therapy has shown a meaningful improvement in ORR.
"The 2021 nucleic acid drug development forum is about to be held in 2021.
It kicked off in Nanjing from May 7th to 8th to welcome the "new era of super drugs",
(MSD) announced that the US Food and Drug Administration (FDA) approved its drug PD-1 therapy Keytruda, trastuzumab, fluoropyrimidine, and platinum-based chemotherapy are used in the first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric cancer or gastroesophageal junction (GEJ) adenocarcinoma patients .
Dr.
Roy Baynes, MSD Research Laboratories Senior Vice President and Chief Medical Officer, Chief Medical Officer, said: "This is the first time the FDA has approved PD-1 therapy in combination with trastuzumab and chemotherapy as the first-line treatment for such patients.
We are very pleased with KEYTRUDA's new first-line combination treatment plan.
Compared with the standard treatment for patients with HER2-positive gastric cancer and GEJ cancer, Keytruda combination therapy has shown a meaningful improvement in ORR.
"The 2021 nucleic acid drug development forum is about to be held in 2021.
It kicked off in Nanjing from May 7th to 8th to welcome the "new era of super drugs",