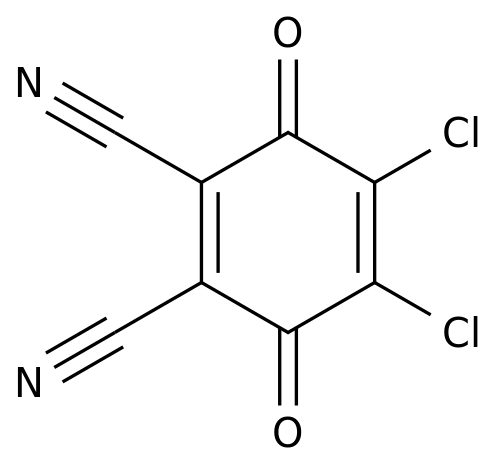
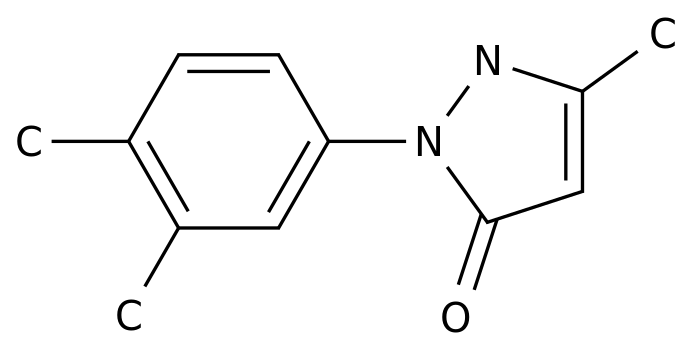
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Medical Network News, March 24.
Recently, my country's independent research and development of the third-generation lung cancer targeted therapy for EGFR (epidermal growth factor receptor) mutations Ivesa (Vometinib mesylate) was awarded the National Medical Products Administration.
Approval for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have experienced disease progression during or after treatment with targeted drugs for EGFR mutations and confirmed to have EGFR T790M mutation-positive.
Recently, my country's independent research and development of the third-generation lung cancer targeted therapy for EGFR (epidermal growth factor receptor) mutations Ivesa (Vometinib mesylate) was awarded the National Medical Products Administration.
Approval for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have experienced disease progression during or after treatment with targeted drugs for EGFR mutations and confirmed to have EGFR T790M mutation-positive.
Aifusha is a national class 1 new drug originally developed in China with independent intellectual property rights.
According to the Chinese Academy of Medical Sciences Tumor Hospital, vice president Professor Shi Yuankai believes Yifu Sha approved only for patients with advanced NSCLC is a domestic Gospel, but also for reducing the mortality of lung cancer is also a great good news.
According to the Chinese Academy of Medical Sciences Tumor Hospital, vice president Professor Shi Yuankai believes Yifu Sha approved only for patients with advanced NSCLC is a domestic Gospel, but also for reducing the mortality of lung cancer is also a great good news.
In recent years, first-generation and second-generation drugs against EGFR targets associated with significantly improved advanced NSCLC without EGFR mutations in disease progression-free survival, but failed to bring significant change to this part of the overall survival of patients with advanced lung cancer, Moreover, patients receiving first-generation EGFR-TKI treatment are more likely to have brain metastases than patients receiving chemotherapy.
Once lung cancer brain metastasis occurs, the median survival time of patients who do not receive any treatment is only 2 months.
Therefore, the control and even prevention of lung cancer brain metastasis has become a medical core to reduce the recurrence and mortality of advanced lung cancer.
Once lung cancer brain metastasis occurs, the median survival time of patients who do not receive any treatment is only 2 months.
Therefore, the control and even prevention of lung cancer brain metastasis has become a medical core to reduce the recurrence and mortality of advanced lung cancer.
Professor Yuankai Shi pointed out that the third-generation EGFR-TKI has been recommended by authoritative lung cancer diagnosis and treatment guidelines in the United States and China as a first-line treatment for EGFR-sensitive mutations and T790M-mutated NSCLC.
The original third-generation EGFR-TKI treatment obtained survival benefits and contributed to reducing the high mortality rate of lung cancer in my country.
The original third-generation EGFR-TKI treatment obtained survival benefits and contributed to reducing the high mortality rate of lung cancer in my country.
It is reported that recently, the Beijing Kangmeng Charity Foundation has launched the Aifusha Patient Assistance Project ("Fuyao" Newborn) to reduce the burden of medication for lung cancer patients in China.
Medical Network News, March 24.
Recently, my country's independent research and development of the third-generation lung cancer targeted therapy for EGFR (epidermal growth factor receptor) mutations Ivesa (Vometinib mesylate) was awarded the National Medical Products Administration.
Approval for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have experienced disease progression during or after treatment with targeted drugs for EGFR mutations and confirmed to have EGFR T790M mutation-positive.
Recently, my country's independent research and development of the third-generation lung cancer targeted therapy for EGFR (epidermal growth factor receptor) mutations Ivesa (Vometinib mesylate) was awarded the National Medical Products Administration.
Approval for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have experienced disease progression during or after treatment with targeted drugs for EGFR mutations and confirmed to have EGFR T790M mutation-positive.
Aifusha is a national class 1 new drug originally developed in China with independent intellectual property rights.
According to the Chinese Academy of Medical Sciences Tumor Hospital, vice president Professor Shi Yuankai believes Yifu Sha approved only for patients with advanced NSCLC is a domestic Gospel, but also for reducing the mortality of lung cancer is also a great good news.
According to the Chinese Academy of Medical Sciences Tumor Hospital, vice president Professor Shi Yuankai believes Yifu Sha approved only for patients with advanced NSCLC is a domestic Gospel, but also for reducing the mortality of lung cancer is also a great good news.
In recent years, first-generation and second-generation drugs against EGFR targets associated with significantly improved advanced NSCLC without EGFR mutations in disease progression-free survival, but failed to bring significant change to this part of the overall survival of patients with advanced lung cancer, Moreover, patients receiving first-generation EGFR-TKI treatment are more likely to have brain metastases than patients receiving chemotherapy.
Once lung cancer brain metastasis occurs, the median survival time of patients who do not receive any treatment is only 2 months.
Therefore, the control and even prevention of lung cancer brain metastasis has become a medical core to reduce the recurrence and mortality of advanced lung cancer.
Once lung cancer brain metastasis occurs, the median survival time of patients who do not receive any treatment is only 2 months.
Therefore, the control and even prevention of lung cancer brain metastasis has become a medical core to reduce the recurrence and mortality of advanced lung cancer.
Professor Yuankai Shi pointed out that the third-generation EGFR-TKI has been recommended by authoritative lung cancer diagnosis and treatment guidelines in the United States and China as a first-line treatment for EGFR-sensitive mutations and T790M-mutated NSCLC.
The original third-generation EGFR-TKI treatment obtained survival benefits and contributed to reducing the high mortality rate of lung cancer in my country.
The original third-generation EGFR-TKI treatment obtained survival benefits and contributed to reducing the high mortality rate of lung cancer in my country.
It is reported that recently, the Beijing Kangmeng Charity Foundation has launched the Aifusha Patient Assistance Project ("Fuyao" Newborn) to reduce the burden of medication for lung cancer patients in China.
Medical Network News, March 24.
Recently, my country's independent research and development of the third-generation lung cancer targeted therapy for EGFR (epidermal growth factor receptor) mutations Ivesa (Vometinib mesylate) was awarded the National Medical Products Administration.
Approval for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have experienced disease progression during or after treatment with targeted drugs for EGFR mutations and confirmed to have EGFR T790M mutation-positive.
Recently, my country's independent research and development of the third-generation lung cancer targeted therapy for EGFR (epidermal growth factor receptor) mutations Ivesa (Vometinib mesylate) was awarded the National Medical Products Administration.
Approval for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have experienced disease progression during or after treatment with targeted drugs for EGFR mutations and confirmed to have EGFR T790M mutation-positive.
Aifusha is a national class 1 new drug originally developed in China with independent intellectual property rights.
According to the Chinese Academy of Medical Sciences Tumor Hospital, vice president Professor Shi Yuankai believes Yifu Sha approved only for patients with advanced NSCLC is a domestic Gospel, but also for reducing the mortality of lung cancer is also a great good news.
Tumor Hospital Tumor Tumor Hospital HospitalAccording to the Chinese Academy of Medical Sciences Tumor Hospital, vice president Professor Shi Yuankai believes Yifu Sha approved only for patients with advanced NSCLC is a domestic Gospel, but also for reducing the mortality of lung cancer is also a great good news.
In recent years, first-generation and second-generation drugs against EGFR targets associated with significantly improved advanced NSCLC without EGFR mutations in disease progression-free survival, but failed to bring significant change to this part of the overall survival of patients with advanced lung cancer, Moreover, patients receiving first-generation EGFR-TKI treatment are more likely to have brain metastases than patients receiving chemotherapy.
Once lung cancer brain metastasis occurs, the median survival time of patients who do not receive any treatment is only 2 months.
Therefore, the control and even prevention of lung cancer brain metastasis has become a medical core to reduce the recurrence and mortality of advanced lung cancer.
Disease disease diseaseOnce lung cancer brain metastasis occurs, the median survival time of patients who do not receive any treatment is only 2 months.
Therefore, the control and even prevention of lung cancer brain metastasis has become a medical core to reduce the recurrence and mortality of advanced lung cancer.
Professor Yuankai Shi pointed out that the third-generation EGFR-TKI has been recommended by authoritative lung cancer diagnosis and treatment guidelines in the United States and China as a first-line treatment for EGFR-sensitive mutations and T790M-mutated NSCLC.
The original third-generation EGFR-TKI treatment obtained survival benefits and contributed to reducing the high mortality rate of lung cancer in my country.
The original third-generation EGFR-TKI treatment obtained survival benefits and contributed to reducing the high mortality rate of lung cancer in my country.
It is reported that recently, the Beijing Kangmeng Charity Foundation has launched the Aifusha Patient Assistance Project ("Fuyao" Newborn) to reduce the burden of medication for lung cancer patients in China.