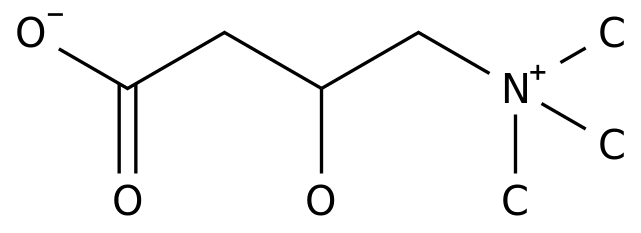
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Malaria is a mosquito-borne parasitic disease that caused approximately 229 million cases and 409,000 deaths in 2019
Child prevention
The most clinically advanced Plasmodium falciparum vaccine RTS,S is a subunit vaccine consisting of a single recombinant protein-Plasmodium falciparum Circumsporium protein (PfCSP), administered together with the adjuvant AS01
The PfSPZ Plasmodium Vaccine uses a different method, including live (metabolically active), non-replicating, radiation-attenuated, sterile, purified, cryopreserved Plasmodium falciparum spores (SPZ)
Initial research showed that among American adults who were not infected with malaria, the VE for a homologous (the parasite strain used for the challenge is the same as the one used for the vaccine) controlled human malaria infection ( CHMI ) is about 60- 100% , up to 14 months.
T cell mediated immunity force is the force necessary to protect the vaccine condition T cell mediated immunity force is a force of a vaccine to provide protection requirement immune
Recently, researchers in 336 Ming 5-12 conducted month-old baby in a multi-arm, randomized, double-blind, placebo-controlled trial to determine PfSPZ vaccine safety under the high spread of malaria in western Kenya environment, tolerance Sex, immunogenicity and efficacy ( NCT02687373 )
Researchers at 336 Ming 5-12 conducted month-old baby in a multi-arm, randomized, double-blind, placebo-controlled trial to determine PfSPZ vaccine safety under the high spread of malaria in western Kenya environment, tolerance, immunogenicity and efficacy ( NCT02687373 researchers in 336 Ming 5-12 were month-old baby in a multi-arm, randomized, double-blind, placebo-controlled trial to determine PfSPZ vaccine high transmission of malaria in western Kenya under the environment Safety, tolerability, immunogenicity and efficacy ( NCT02687373 )
5 5 6 The vaccine is well tolerated.
Time to first appearance of parasitemia
Time of first appearance of parasitemia Time of first appearance of parasitemiaUnfortunately, any dose group has no obvious protective effect against Plasmodium falciparum infection at 6 months ( VE=-6.
Any dose group at 6 Shi months for P.
T cell responses in all dose groups were undetectable
These data indicate that the T cell immunity induced by the PfSPZ vaccine is age-related and may be affected by the frequency of Vδ2+Vγ9+ T cells
PfSPZ vaccine-induced T cell immunity with age-related PfSPZ vaccine-induced T cell immunity with age-related
Original source:
Original source:Martina Oneko et al.
Martina Oneko et al.
Leave a message here