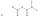-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
For more drug research information, it’s all in investing in Upacicalcet (UPASITA®) developed by Ecosanhe Chemical Co.
, Ltd.
, which is a CaSR agonist.
It was approved for marketing by Japan PMDA on June 23, 2021 for the treatment of secondary hemodialysis patients.
Hyperparathyroidism [1]
.
Renal hyperparathyroidism is a common complication of chronic kidney disease, wherein the parathyroid hormone levels secondary to calcium, phosphate and vitamin D disorders increases
.
There are approximately 660,000 patients with end-stage renal disease who rely on dialysis in the United States.
Some patients can improve their condition by using vitamin D analogs, phosphate binders or calcimimetic agents, but some patients still need parathyroidectomy to reduce their condition and The impact of sequelae [2]
.
The approval of Upacicalcet is based on the results of two phase III clinical trials conducted in Japan, one of which included a total of 153 patients with secondary hyperparathyroidism on hemodialysis who used Upacicalcet and 3 times a week after dialysis.
Placebo, after 24 weeks, the proportion of patients with serum parathyroid hormone (iPTH) concentration ≥60 pg/ml and ≤240 pg/ml in the Upacicalcet treatment group was significantly higher than that of the placebo group (67.
0% vs 8.
0%, p <0.
001)
.
In another single-arm trial conducted in 157 patients, 94.
2% of patients had serum iPTH concentrations ≥60 pg/ml and ≤240 pg/ml after 52 weeks of treatment [3]
.
For details of Upacicalcet and the competitive landscape of CaSR agonist chemical drugs, see the following figure: (click to enlarge) You can also click the link below to get the corresponding PDF HD version: https://data.
pharmacodia.
com/v3/insight/#/ListPdf? tag=2Reference materials 1.
Drug crossing data https://data.
pharmacodia.
com 2.
Noah K Yuen, Shubha Ananthakrishnan, Michael J Campbell.
Hyperparathyroidism of Renal Disease.
The Permanente Journal.
2016 Jul.
DOI: 10.
7812/TPP/15 -127.
3.
PMDA drug insert: https://