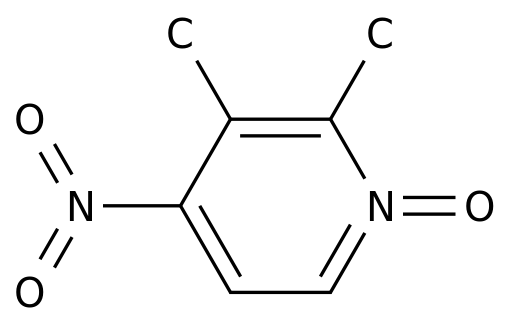
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Medical Network, July 13th.
The Global Alliance for Vaccines and Immunization (GAVI) issued a press release on the 12th, announcing that it has signed pre-purchase agreements with China National Pharmaceutical Group and Kexing.
This means that Sinopharm Vaccine and Kexing Vaccine have entered the "new crown pneumonia vaccine".
Implementation Plan” (COVAX) Vaccine Bank, and the vaccine can be supplied to COVAX from July for the prevention and control of the new crown epidemic in developing countries
.
COVAX is led by the World Health Organization and the Global Alliance for Vaccines and Immunization
.
According to the press release, considering that these two vaccines have entered the WHO emergency use list, they can be immediately supplied to COVAX participants
.
According to the agreement, the two Chinese companies will be able to supply COVAX with 110 million doses of the new crown vaccine before the end of October this year, with subsequent long-term supply
.
The agreement between the Global Alliance for Vaccines and Immunizations and Chinese companies came at a time when the world, especially developing countries, urgently needed to expand vaccination rates and curb the spread of the new coronavirus
.
Seth Berkeley, CEO of the Global Alliance for Vaccines and Immunization, said in the communiqué: “I welcome today’s agreement, which allows COVAX participants to obtain the vaccine immediately
.
” A person from the Permanent Mission of China to the United Nations in Geneva said that the two companies have a global The signing of the agreement by the Alliance for Vaccines and Immunization is an important manifestation of China's practical actions in fulfilling its promise as a "global public product" for vaccines
.
The Chinese government has been actively encouraging and supporting Chinese vaccine research and development companies to participate in COVAX and provide vaccines to developing countries
.
China currently has a number of new crown vaccines that have obtained emergency use licenses in the country, and there are also a number of vaccines that are in the clinical trial stage
.
In addition, many Chinese vaccine companies have expressed their positive willingness to join COVAX.
We look forward to more Chinese vaccines entering the WHO emergency use list as soon as possible, and being selected into the COVAX vaccine library as soon as possible, making positive contributions to global solidarity in the fight against the epidemic
.
The Global Alliance for Vaccines and Immunization (GAVI) issued a press release on the 12th, announcing that it has signed pre-purchase agreements with China National Pharmaceutical Group and Kexing.
This means that Sinopharm Vaccine and Kexing Vaccine have entered the "new crown pneumonia vaccine".
Implementation Plan” (COVAX) Vaccine Bank, and the vaccine can be supplied to COVAX from July for the prevention and control of the new crown epidemic in developing countries
.
COVAX is led by the World Health Organization and the Global Alliance for Vaccines and Immunization
.
According to the press release, considering that these two vaccines have entered the WHO emergency use list, they can be immediately supplied to COVAX participants
.
According to the agreement, the two Chinese companies will be able to supply COVAX with 110 million doses of the new crown vaccine before the end of October this year, with subsequent long-term supply
.
The agreement between the Global Alliance for Vaccines and Immunizations and Chinese companies came at a time when the world, especially developing countries, urgently needed to expand vaccination rates and curb the spread of the new coronavirus
.
Seth Berkeley, CEO of the Global Alliance for Vaccines and Immunization, said in the communiqué: “I welcome today’s agreement, which allows COVAX participants to obtain the vaccine immediately
.
” A person from the Permanent Mission of China to the United Nations in Geneva said that the two companies have a global The signing of the agreement by the Alliance for Vaccines and Immunization is an important manifestation of China's practical actions in fulfilling its promise as a "global public product" for vaccines
.
The Chinese government has been actively encouraging and supporting Chinese vaccine research and development companies to participate in COVAX and provide vaccines to developing countries
.
China currently has a number of new crown vaccines that have obtained emergency use licenses in the country, and there are also a number of vaccines that are in the clinical trial stage
.
In addition, many Chinese vaccine companies have expressed their positive willingness to join COVAX.
We look forward to more Chinese vaccines entering the WHO emergency use list as soon as possible, and being selected into the COVAX vaccine library as soon as possible, making positive contributions to global solidarity in the fight against the epidemic
.