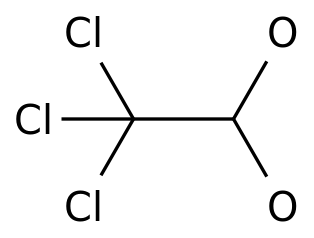
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Left atrial appendage closure (LAAC) is an effective measure to prevent cardiogenic stroke in patients with atrial fibrillation (atrial fibrillation), and it is gradually being used in more and more clinical applications
.
In recent years, non-vitamin K antagonist oral anticoagulants (NOAC) have become more and more widely used in patients with non-valvular atrial fibrillation
.
There is a lack of randomized controlled studies with long-term follow-up in the LAAC field, and published studies focus on comparing LAAC and warfarin
.
For high-risk patients with atrial fibrillation, how to choose between LAAC and NOAC treatment is a clinical issue that needs to be discussed urgently
.
The PRAGUE-17 trial is the first clinical study to compare LAAC and NOAC.
The 19.
9-month initial research results of the trial previously published indicate that for patients with high-risk atrial fibrillation, left atrial appendage occlusion (LAAC) prevents major adverse cardiovascular events and nerves The efficacy of systemic events is not inferior to NOAC, and the incidence of bleeding events in the LAAC group is not higher than that in the NOAC group
.
Recently, the 4-year follow-up results of the PRAGUE-17 trial were published in the Journal of the American College of Cardiology (JACC)
.
Research Methods The PRAGUE-17 study is a prospective, multicenter, open-label randomized non-inferiority study, which aims to compare the efficacy and safety of LAAC and NOAC in high-risk patients with atrial fibrillation
.
The researchers enrolled 402 high-risk patients with atrial fibrillation (with a history of cardiogenic thromboembolism or clinically relevant bleeding, or CHA2DS2-VASc score ≥ 3 and HAS-BLED score ≥ 2) from 10 heart centers in the Czech Republic.
Randomly assigned to the LAAC group (using WATCHMAN or Amulet left atrial appendage occluder) and NOAC group (95.
5% of Apixaban) in a 1:1 ratio
.
The primary endpoint was a composite endpoint of cardiogenic thromboembolic events (stroke, transient ischemic attack or systemic embolism), cardiovascular death, clinically related bleeding, and surgery/device-related complications (LAAC group only)
.
According to the standards of the International Society for Thrombosis and Hemostasis (ISTH), clinically relevant bleeding includes clinically relevant major bleeding and clinically relevant non-major bleeding
.
Major bleeding includes hemoglobin drop of ≥2.
0 g/dL within 24 hours, infusion of ≥2 units of red blood cell suspension, bleeding in key parts, or fatal bleeding
.
Clinically relevant non-major bleeding refers to bleeding that requires hospitalization or invasive surgery, but does not meet the main criteria for ISTH
.
Secondary endpoints were cardiovascular death, all strokes/transient ischemic attacks, clinically relevant bleeding, and non-surgical clinically relevant bleeding
.
The main analysis is the improvement intention analysis (mITT)
.
Research results The average age of the subjects was 73.
3, males accounted for 65.
7%, the average CHA2DS2-VASc score was 4.
7 points (more than 25% of patients had CHA2DS2-VASc score> 6 points), and the average HAS-BLED score was 3.
1 points
.
Among them, 35.
3% of patients had a history of cardiogenic thromboembolism, and 47.
8% of patients had a history of clinically relevant bleeding
.
The median follow-up time was 3.
5 years.
According to the modified intention analysis, the primary composite endpoint of the LAAC group was not inferior to that of the NOAC group (partially distributed hazard ratio [sHR], 0.
81; 95% CI, 0.
56-1.
18; p=0.
27; non-inferior) Effectiveness p value = 0.
006)
.
Figure 1 The sHR and 95% CI of the primary composite endpoints of the study are: cardiovascular death (sHR, 0.
68; 95% CI, 0.
39-1.
20; p=0.
19), all strokes/TIA (sHR, 1.
14; 95 %CI, 0.
56-2.
30; p=0.
72), clinically related bleeding (sHR, 0.
75; 95%CI, 0.
44-1.
27; p=0.
28), non-surgical clinically related bleeding (sHR, 0.
55; 95%CI, 0.
31-0.
97 ; P=0.
039)
.
Figure 2 Secondary endpoint of the study Note: TIA, transient ischemic attack
.
Following the study protocol analysis [sHR 0.
80 (95% CI 0.
54-1.
18), p=0.
25] and receiving treatment analysis [sHR 0.
82 (95% CI 0.
56-1.
20), p=0.
30], the primary composite endpoint results were similar
.
Conclusion The long-term follow-up results of the PRAGUE-17 study showed that for patients with nonvalvular atrial fibrillation at high risk of stroke and bleeding, cardiogenic thromboembolic events, cardiovascular deaths, clinically related bleeding, and surgical/device-related complications in the LAAC treatment group The composite endpoint is not inferior to the NOAC treatment group
.
Compared with the NOAC treatment group, the non-surgical clinically relevant bleeding rate of LAAC was significantly reduced
.
Discussion Some researchers believe that compared with NOAC, the long-term non-surgical clinically relevant bleeding rate of LAAC is significantly reduced, and LAAC can be considered as a non-drug alternative strategy for long-term anticoagulation therapy in patients with high-risk atrial fibrillation
.
Dr.
Merchant FM pointed out that the PRAGUE-17 study did not clearly report long-term device-related adverse reactions in the LAAC group, such as device-related thrombosis (DRT) and occluder marginal shunt (PDL).
These adverse reactions may increase systemic embolism (SSE) Risk
.
In addition, due to the impact of the new coronary pneumonia epidemic, many patients failed to complete the transesophageal echocardiography (TEE) follow-up, and it was impossible to clarify the surface thrombosis and marginal shunt of the occluder
.
The PRAGUE-17 study has certain limitations, but until more reliable data is obtained, the long-term results of the study have certain guiding value for the treatment of high-risk atrial fibrillation patients
.
Currently, two large randomized trials comparing LAAC and NOAC are underway, let us wait and see
.
References: 1.
Osmancik P, Herman D, Neuzil P, et al.
Left Atrial Appendage Closure versus Non-Warfarin Oral Anticoagulation in Atrial Fibrillation: 4-Year Outcomes of PRAGUE-17.
J Am Coll Cardiol.
2021 Oct 27: S0735 -1097(21)07895-5.
doi: 10.
1016/j.
jacc.
2021.
10.
023.
Epub ahead of print.
PMID: 34748929.
2.
Merchant FM.
Does Percutaneous Left Atrial Appendage Closure Stand the Test of Time? J Am Coll Cardiol.
2021 Oct 25: S0735-1097(21)07894-3.
doi:10.
1016/j.
jacc.
2021.
10.
022.
Epub ahead of print.
PMID: 34748930.
.
In recent years, non-vitamin K antagonist oral anticoagulants (NOAC) have become more and more widely used in patients with non-valvular atrial fibrillation
.
There is a lack of randomized controlled studies with long-term follow-up in the LAAC field, and published studies focus on comparing LAAC and warfarin
.
For high-risk patients with atrial fibrillation, how to choose between LAAC and NOAC treatment is a clinical issue that needs to be discussed urgently
.
The PRAGUE-17 trial is the first clinical study to compare LAAC and NOAC.
The 19.
9-month initial research results of the trial previously published indicate that for patients with high-risk atrial fibrillation, left atrial appendage occlusion (LAAC) prevents major adverse cardiovascular events and nerves The efficacy of systemic events is not inferior to NOAC, and the incidence of bleeding events in the LAAC group is not higher than that in the NOAC group
.
Recently, the 4-year follow-up results of the PRAGUE-17 trial were published in the Journal of the American College of Cardiology (JACC)
.
Research Methods The PRAGUE-17 study is a prospective, multicenter, open-label randomized non-inferiority study, which aims to compare the efficacy and safety of LAAC and NOAC in high-risk patients with atrial fibrillation
.
The researchers enrolled 402 high-risk patients with atrial fibrillation (with a history of cardiogenic thromboembolism or clinically relevant bleeding, or CHA2DS2-VASc score ≥ 3 and HAS-BLED score ≥ 2) from 10 heart centers in the Czech Republic.
Randomly assigned to the LAAC group (using WATCHMAN or Amulet left atrial appendage occluder) and NOAC group (95.
5% of Apixaban) in a 1:1 ratio
.
The primary endpoint was a composite endpoint of cardiogenic thromboembolic events (stroke, transient ischemic attack or systemic embolism), cardiovascular death, clinically related bleeding, and surgery/device-related complications (LAAC group only)
.
According to the standards of the International Society for Thrombosis and Hemostasis (ISTH), clinically relevant bleeding includes clinically relevant major bleeding and clinically relevant non-major bleeding
.
Major bleeding includes hemoglobin drop of ≥2.
0 g/dL within 24 hours, infusion of ≥2 units of red blood cell suspension, bleeding in key parts, or fatal bleeding
.
Clinically relevant non-major bleeding refers to bleeding that requires hospitalization or invasive surgery, but does not meet the main criteria for ISTH
.
Secondary endpoints were cardiovascular death, all strokes/transient ischemic attacks, clinically relevant bleeding, and non-surgical clinically relevant bleeding
.
The main analysis is the improvement intention analysis (mITT)
.
Research results The average age of the subjects was 73.
3, males accounted for 65.
7%, the average CHA2DS2-VASc score was 4.
7 points (more than 25% of patients had CHA2DS2-VASc score> 6 points), and the average HAS-BLED score was 3.
1 points
.
Among them, 35.
3% of patients had a history of cardiogenic thromboembolism, and 47.
8% of patients had a history of clinically relevant bleeding
.
The median follow-up time was 3.
5 years.
According to the modified intention analysis, the primary composite endpoint of the LAAC group was not inferior to that of the NOAC group (partially distributed hazard ratio [sHR], 0.
81; 95% CI, 0.
56-1.
18; p=0.
27; non-inferior) Effectiveness p value = 0.
006)
.
Figure 1 The sHR and 95% CI of the primary composite endpoints of the study are: cardiovascular death (sHR, 0.
68; 95% CI, 0.
39-1.
20; p=0.
19), all strokes/TIA (sHR, 1.
14; 95 %CI, 0.
56-2.
30; p=0.
72), clinically related bleeding (sHR, 0.
75; 95%CI, 0.
44-1.
27; p=0.
28), non-surgical clinically related bleeding (sHR, 0.
55; 95%CI, 0.
31-0.
97 ; P=0.
039)
.
Figure 2 Secondary endpoint of the study Note: TIA, transient ischemic attack
.
Following the study protocol analysis [sHR 0.
80 (95% CI 0.
54-1.
18), p=0.
25] and receiving treatment analysis [sHR 0.
82 (95% CI 0.
56-1.
20), p=0.
30], the primary composite endpoint results were similar
.
Conclusion The long-term follow-up results of the PRAGUE-17 study showed that for patients with nonvalvular atrial fibrillation at high risk of stroke and bleeding, cardiogenic thromboembolic events, cardiovascular deaths, clinically related bleeding, and surgical/device-related complications in the LAAC treatment group The composite endpoint is not inferior to the NOAC treatment group
.
Compared with the NOAC treatment group, the non-surgical clinically relevant bleeding rate of LAAC was significantly reduced
.
Discussion Some researchers believe that compared with NOAC, the long-term non-surgical clinically relevant bleeding rate of LAAC is significantly reduced, and LAAC can be considered as a non-drug alternative strategy for long-term anticoagulation therapy in patients with high-risk atrial fibrillation
.
Dr.
Merchant FM pointed out that the PRAGUE-17 study did not clearly report long-term device-related adverse reactions in the LAAC group, such as device-related thrombosis (DRT) and occluder marginal shunt (PDL).
These adverse reactions may increase systemic embolism (SSE) Risk
.
In addition, due to the impact of the new coronary pneumonia epidemic, many patients failed to complete the transesophageal echocardiography (TEE) follow-up, and it was impossible to clarify the surface thrombosis and marginal shunt of the occluder
.
The PRAGUE-17 study has certain limitations, but until more reliable data is obtained, the long-term results of the study have certain guiding value for the treatment of high-risk atrial fibrillation patients
.
Currently, two large randomized trials comparing LAAC and NOAC are underway, let us wait and see
.
References: 1.
Osmancik P, Herman D, Neuzil P, et al.
Left Atrial Appendage Closure versus Non-Warfarin Oral Anticoagulation in Atrial Fibrillation: 4-Year Outcomes of PRAGUE-17.
J Am Coll Cardiol.
2021 Oct 27: S0735 -1097(21)07895-5.
doi: 10.
1016/j.
jacc.
2021.
10.
023.
Epub ahead of print.
PMID: 34748929.
2.
Merchant FM.
Does Percutaneous Left Atrial Appendage Closure Stand the Test of Time? J Am Coll Cardiol.
2021 Oct 25: S0735-1097(21)07894-3.
doi:10.
1016/j.
jacc.
2021.
10.
022.
Epub ahead of print.
PMID: 34748930.