-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read and refer to the progress of MET targets in lung cancer
.
In 2021, the first year of MET in China, the original drug research and development led by Professor Lu Shun of the Chest Hospital Affiliated to Shanghai Jiaotong University was successfully approved for marketing in China, becoming the first class 1 innovative drug MET targeted drug in China
.
With the availability of MET-targeted drugs, the improvement of related diagnosis and treatment detection technologies, and the continuous expansion of new drug research and development, the concept and path of MET pathway precision diagnosis and treatment have become increasingly clear, and the field of rare MET targets in lung cancer has ushered in many new progress and new phenomena.
, new breakthroughs
.
On January 21, 2022, the "China Lung Cancer MET Target Progress Inventory Cloud Summit" hosted by the "Medical Community" was held in Shanghai, and was simultaneously broadcast live on the "Medical Community Doctor Station APP" platform
.
The conference was chaired by Professor Liao Meilin and Professor Lu Shun from the Chest Hospital of Shanghai Jiaotong University
.
At the meeting, Professor Lu Shun reviewed the research progress of MET-targeted therapy, and looked forward to the development and exploration direction of future clinical diagnosis and treatment
.
In addition, a number of top leaders in the field of lung cancer in China shared experience and discussed hot topics on MET-targeted diagnosis and treatment
.
Let us take a look at the exciting content! Prof.
Meilin Liao and Prof.
Shun Lu, the chairmen of the conference, shared the academic essence of MET - MET-targeted therapy research progress and outlook Prof.
Shun Lu Abnormal activation of the MET pathway can lead to the occurrence and development of tumors, the main forms include: MET exon 14 skipping mutation , MET amplification, MET protein overexpression, MET kinase domain mutations, and MET fusions
.
At this conference, Professor Lu Shun first reviewed the discovery process of MET, pointed out that 38 years since the first discovery in 1984, the exploration of MET has ushered in a new era, and focused on sharing the clinical studies of MET exon 14 skipping mutation, MET amplification and overexpression.
Treatment progress and outlook
.
1.
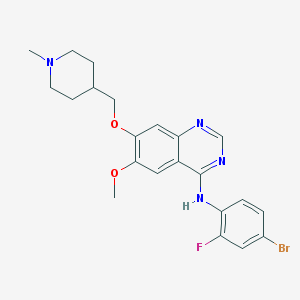
MET exon 14 skipping mutation: "Breaking out of the chrysalis", sivotinib has become a new hope for the treatment of Chinese patients At present, TKIs used for MET exon 14 skipping mutation at home and abroad include sivotinib, Capmatinib, Tepotinib, and crizole Amivantamab, of which savatinib is the first and currently the only MET-TKI approved in China, has conducted the only study to date that includes 100% of the Chinese population
.
The results of the phase II study of sevolitinib in the treatment of MET exon 14 skipping NSCLC in China showed that MET exon 14 skipping NSCLC had a rapid onset of treatment and showed good and durable tumor remission
.
The independent review committee (IRC)-assessed objective response rate (ORR) in the efficacy-evaluable set was 49.
2%, disease control rate (DCR) was 93.
4%, and a duration of response (DoR) of 9.
6 months was achieved; median none Progression survival (PFS) was 6.
8 months, and median overall survival (OS) was 12.
5 months
.
In addition, the treatment of patients with different MET exon 14 skipping mutations and MET amplification with sevolitinib did not affect the efficacy
.
Moreover, the safety of savatinib was good, and no interstitial pneumonia occurred
.
Professor Lu Shun also demonstrated the special clinical use of sevolitinib in the treatment of MET exon 14 skipping mutation NSCLC through 2 cases
.
One of them is an 89-year-old woman with right upper lobe poorly differentiated carcinoma (c-T4N3M1c, stage IVB) diagnosed with MET exon 14 skipping mutation in July 2021, and she was given oral savatinib 200 mg in August 2021 25-day efficacy assessment as partial response (PR)
.
Another case was a liver transplant patient who was diagnosed with MET exon 14 skipping mutation of left upper lung adenocarcinoma (c-T3N3M1a, stage IV) in September 2021, and was given oral savatinib 200mg qd and gradually increased to 3 weeks.
600mg qd
.
Likewise, the Nov.
9, 2021 efficacy assessment was PR
.
Currently, a phase IIIb confirmatory clinical study (registration number: CTR20211151) of sevolitinib in the treatment of patients with locally advanced or metastatic NSCLC with MET exon 14 skipping mutation with Professor Lu Shun as the main PI is ongoing
.
This is a single-arm, open-label, multicenter phase III study with a planned enrollment of 163 patients divided into 2 cohorts
.
Cohort 1 included patients with disease progression or toxicity intolerance after previous platinum-containing chemotherapy regimens, and cohort 2 included patients who had not received any prior systemic antitumor drug therapy for advanced disease
.
The primary endpoint was IRC-assessed ORR
.
2.
MET amplification and overexpression: There is limited clinical treatment experience, and many explorations are initiating MET amplification as both a primary driver gene and a secondary/co-driver gene
.
MET amplification is one of the important mechanisms of EGFR-TKI resistance
.
Currently, treatment options for MET amplification-driven EGFR-TKI-resistant NSCLC are being explored
.
Amivantamab combined with lazertinib showed a trend of durable remission, and the safety was controllable; Capmatinib combined with gefitinib, Tepotinib combined with gefitinib showed good efficacy; savatinib opened the first combined osimertinib treatment of MET expansion A study of NSCLC (TATTON), and found that regardless of T790M status, savatinib + osimertinib has good and durable anti-tumor activity
.
Data from two expansion cohorts (B and D) of the TATTON study have been published
.
Among the 138 patients included in the Part B cohort, 69 patients had disease progression after receiving third-generation EGFR-TKI therapy (B1), 51 patients had not received third-generation EGFR-TKI therapy before and were T790M negative (B2), and 18 patients were previously Third-generation EGFR-TKI-naïve and T790M-positive (B3); Part D cohort included 42 previously third-generation EGFR-TKI-naïve and T790M-negative patients who received once-daily oral osimertinib + sivotinib combination therapy
.
The results showed that the ORR of parts B1, B2 and B3 were 33%, 65% and 67%, DCR was 75%, 88% and 100%, respectively, and the median PFS was 5.
5 months, 9.
1 months and 11.
1 months, respectively ; Part D ORR was 62%, DCR was 93%, and median PFS was 9.
0 months
.
Currently, more sevolitinib + osimertinib combination therapy options are being explored, including evaluating osimertinib combined with savatinib in the treatment of EGFRm and MET+ locally advanced or metastatic disease that has progressed after osimertinib treatment Phase II, Single-Arm SAVANNAH Study of Efficacy in Patients with NSCLC, Phase III SACHI Study of Cavatinib and Osimertinib Versus Pemetrexed and Platinum in NSCLC with MET Amplification Progressing After EGFR-TKI Therapy (China Registry Study), and Phase III SAFFRON Study (Global Registry Study) of savatinib combined with osimertinib versus chemotherapy in NSCLC with MET amplification/overexpression that has progressed after osimertinib treatment
.
Primary MET amplification, MET amplification and EGFR co-driving, and MET fusion currently have limited clinical treatment experience, and a number of explorations are being initiated, including sevolitinib combined with durvalumab in the treatment of EGFR wild-type with MET abnormalities in China The locally advanced or metastatic NSCLC study (SOUND), and the phase III study of savatinib plus osimertinib versus placebo plus osimertinib in the first-line treatment of EGFRm and MET overexpressing advanced NSCLC (SANOVO)
.
let us wait and see! Based on the present, looking to the future - MET targeted diagnosis and treatment experience sharing and hot spot discussions , and start a hot discussion
.
1.
Manage adverse reactions and optimize clinical decision-making In the first part of the discussion session, Professor Dong Xiaorong of Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Professor Jian Hong of Shanghai Jiaotong University Affiliated Chest Hospital, Professor Li Ziming of Shanghai Jiaotong University Affiliated Chest Hospital, Professor Yang Jinji from Guangdong Provincial People's Hospital and Professor Zhao Jun from Peking University Cancer Hospital had a heated discussion on the following three topics: What are the adverse reactions that you are more concerned about in the clinical application of savatinib? What is your experience with the management and dose adjustment of savatinib-related AEs? When encountering patients with other MET abnormalities (MET amplification/overexpression/fusion, etc.
) other than MET exon 14 skipping mutation, how would you consider the treatment options? Prof.
Zhao Jun, Prof.
Dong Xiaorong and Prof.
Yang Jinji▎Professor Zhao Jun: Edema and abnormal liver function are common toxicities during the clinical use of sevolitinib.
One patient developed allergic toxicity, including fever and rash.
It may also be less reported in the past
.
However, in other patients, the overall safety was good and generally controllable
.
Clinically, in addition to the detection of DNA, RNA-based detection is also a challenge, including the extraction of specimens
.
In addition, immunohistochemistry (IHC) is cheap and convenient in the detection of MET.
The detection and application of IHC for MET overexpression is also worthy of our attention in the future
.
▎Professor Dong Xiaorong: After the launch of savatinib, we have also applied it in the clinic for the three types of MET abnormalities (overexpression/amplification/exon 14 skipping mutation)
.
In terms of adverse reactions, for example, a 50-year-old patient developed high fever (39°C-40°C) and grade 1-2 edema after taking savatinib.
After symptomatic treatment, the fever recurred, and after drug withdrawal Symptoms disappeared
.
The patient's treatment effect achieved partial remission
.
Subsequently, we repeatedly adjusted the dose of sivotinib, but there was still some fever
.
Therefore, whether the cause of fever with savatinib is related to the patient's constitution still needs our attention and discussion
.
▎Professor Yang Jinji: For patients with EGFR mutations who are resistant to targeted therapy, MET fusion will also occur.
Whether the corresponding targeted therapy can be used remains to be determined
.
In addition, based on the patients with MET fusion after DNA-NGS detection, preliminary observations show that the efficacy of dual-target therapy in this population is limited
.
Whether it needs to be further verified by RNA-NGS remains to be further explored
.
Professor Jian Hong▎Professor Jianhong: At present, sevolitinib is mainly used in patients with MET14 exon skipping mutation, and fever is an adverse reaction worthy of attention
.
In addition, for patients with MET amplification after EGFR-TKI resistance, I think detection is very important and can be screened by FISH
.
For this population, sevolitinib 300 mg was well tolerated
.
Regarding MET overexpression, more research is needed to confirm the efficacy of targeted therapy, but the treatment options for these patients are limited, and targeted therapy may be a "breakthrough"
.
Professor Li Ziming▎Professor Li Ziming: For patients with MET exon 14 skipping mutation who developed abnormal liver function after taking 600mg of sevolitinib, reducing the dose of sevolitinib to 400mg, coupled with liver protection therapy, may make liver function worse back to normal
.
Most MET amplification or overexpression occurs after secondary drug resistance, which may be different from primary MET amplification or overexpression, and more research is needed to explore
.
▎Professor Lu Shun: For allergic reactions, such as fever and rash, we use hormonal drugs, and the symptoms are generally relieved
.
In addition, liver function damage is also worthy of our attention, especially when abnormal bilirubin and albumin indicators occur, reducing the dose of savatinib is a solution that can be considered
.
At present, the dose of sivotinib is mainly judged according to body weight in clinical practice, and experience and experience still need to be summarized in clinical practice in the future
.
2.
Precise treatment, detection first As a guiding speech, Professor Zhou Xiaoyan of Fudan University Affiliated Cancer Hospital first introduced the main points of clinical practice of MET detection
.
Among them, the commonly used detection methods for MET exon 14 skipping mutation are RT-qPCR and NGS, the commonly used detection methods for MET gene amplification are FISH and NGS, and the detection method for MET protein overexpression is IHC
.
Different detection methods have their own advantages and disadvantages, and the appropriate detection method should be selected according to the actual situation in clinical practice
.
Next, Professor Zhou Xiaoyan, Professor Ai Xinghao of Shanghai Jiaotong University Chest Hospital, Professor Fan Min of Fudan University Cancer Hospital, Professor Gao Beili of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Professor Han Yuchen of Shanghai Jiaotong University Chest Hospital, Fudan University Affiliated Professor Hu Jie of Zhongshan Hospital and Professor Wang Jialei of Fudan University Affiliated Cancer Hospital discussed the following questions: How to accurately detect MET14 exon skipping mutation? Do you think RT-qPCR is reliable for detecting MET14 exon skipping mutations? RNA-based assays have higher detection capabilities, but RNA samples are easily degraded.
How to efficiently achieve tissue extraction from patients? May I ask what kind of patients are considered clinically for MET amplification testing? What testing method will be chosen? Which method do you think needs improvement to better detect MET gene amplification? At present, there is no unified standard for the cutoff value of different MET amplification detection methods.
How do you consider the timing of starting treatment? At what threshold is MET protein overexpression clinically significant? Whether the clinic will consider initiating treatment
.
Professor Ai Xinghao▎Professor Ai Xinghao: Clinically, the incidence of MET amplification detected in EGFR-TKI-resistant patients does not reach the incidence reported in the literature
.
In addition, there are still doubts about the correlation of MET amplification, FISH and NGS detection results, and the effect of local amplification and multiligand amplification on the efficacy of MET-TKI
.
Professor Han Yuchen▎Professor Han Yuchen: For non-small cell lung cancer, we emphasize multi-gene testing
.
For patients with MET amplifications that are not abundant after NGS detection, FISH verification may be necessary
.
In terms of optimizing the process, we look forward to the release of relevant consensus
.
Data is still needed to determine whether immunohistochemistry can be used as MET amplification
.
Professor Fan Min▎Professor Fan Min: RNA-NGS is often used in the inspection of MET in our hospital, but it is highly dependent on the platform
.
In the past, MET overexpression was considered to be less able to guide clinical practice, and the criteria for interpreting MET amplification still need to be clarified
.
Prof.
Beili Gao ▎ Prof.
Beili Gao: At present, clinically, it is still difficult to start MET-TKI therapy in patients with MET overexpression
.
In addition, whether immunohistochemistry can be directly qualitative or not must be verified by NGS, and how to optimize the treatment plan with existing treatment methods is also a clinical problem that needs to be solved
.
Prof.
Hu Jie ▎ Prof.
Hu Jie: For the MET signaling pathway, the MET exon 14 skipping mutation has been well defined, but the MET amplification is still controversial
.
For example, DNA-level MET amplification detection includes FISH and NGS, but the detection results of different methods are different.
Will this affect the treatment effect? As a clinician, I want patients to receive precise care without over-testing and under-treatment
.
Professor Wang Jialei▎Professor Wang Jialei: The situation of abnormal MET pathway is very complicated.
In terms of treatment, how to optimize the treatment sequence and how to balance the efficacy and safety in clinical practice; in terms of detection, how to solve the problems of tumor heterogeneity and cross-mutation, etc.
resolve
.
Summary: Professor Liao Meilin concluded at the end that this meeting brought together pathology departments and clinicians, and promoted exchanges and learning between the two departments
.
In addition, as a big country in lung cancer, China is in urgent need of its own innovative drugs.
It is hoped that more innovative drugs like savatinib will be developed and marketed in the future to provide more clinical solutions
.
*This article is only used to provide scientific information to medical professionals and does not represent the views of this platform
.
In 2021, the first year of MET in China, the original drug research and development led by Professor Lu Shun of the Chest Hospital Affiliated to Shanghai Jiaotong University was successfully approved for marketing in China, becoming the first class 1 innovative drug MET targeted drug in China
.
With the availability of MET-targeted drugs, the improvement of related diagnosis and treatment detection technologies, and the continuous expansion of new drug research and development, the concept and path of MET pathway precision diagnosis and treatment have become increasingly clear, and the field of rare MET targets in lung cancer has ushered in many new progress and new phenomena.
, new breakthroughs
.
On January 21, 2022, the "China Lung Cancer MET Target Progress Inventory Cloud Summit" hosted by the "Medical Community" was held in Shanghai, and was simultaneously broadcast live on the "Medical Community Doctor Station APP" platform
.
The conference was chaired by Professor Liao Meilin and Professor Lu Shun from the Chest Hospital of Shanghai Jiaotong University
.
At the meeting, Professor Lu Shun reviewed the research progress of MET-targeted therapy, and looked forward to the development and exploration direction of future clinical diagnosis and treatment
.
In addition, a number of top leaders in the field of lung cancer in China shared experience and discussed hot topics on MET-targeted diagnosis and treatment
.
Let us take a look at the exciting content! Prof.
Meilin Liao and Prof.
Shun Lu, the chairmen of the conference, shared the academic essence of MET - MET-targeted therapy research progress and outlook Prof.
Shun Lu Abnormal activation of the MET pathway can lead to the occurrence and development of tumors, the main forms include: MET exon 14 skipping mutation , MET amplification, MET protein overexpression, MET kinase domain mutations, and MET fusions
.
At this conference, Professor Lu Shun first reviewed the discovery process of MET, pointed out that 38 years since the first discovery in 1984, the exploration of MET has ushered in a new era, and focused on sharing the clinical studies of MET exon 14 skipping mutation, MET amplification and overexpression.
Treatment progress and outlook
.
1.
MET exon 14 skipping mutation: "Breaking out of the chrysalis", sivotinib has become a new hope for the treatment of Chinese patients At present, TKIs used for MET exon 14 skipping mutation at home and abroad include sivotinib, Capmatinib, Tepotinib, and crizole Amivantamab, of which savatinib is the first and currently the only MET-TKI approved in China, has conducted the only study to date that includes 100% of the Chinese population
.
The results of the phase II study of sevolitinib in the treatment of MET exon 14 skipping NSCLC in China showed that MET exon 14 skipping NSCLC had a rapid onset of treatment and showed good and durable tumor remission
.
The independent review committee (IRC)-assessed objective response rate (ORR) in the efficacy-evaluable set was 49.
2%, disease control rate (DCR) was 93.
4%, and a duration of response (DoR) of 9.
6 months was achieved; median none Progression survival (PFS) was 6.
8 months, and median overall survival (OS) was 12.
5 months
.
In addition, the treatment of patients with different MET exon 14 skipping mutations and MET amplification with sevolitinib did not affect the efficacy
.
Moreover, the safety of savatinib was good, and no interstitial pneumonia occurred
.
Professor Lu Shun also demonstrated the special clinical use of sevolitinib in the treatment of MET exon 14 skipping mutation NSCLC through 2 cases
.
One of them is an 89-year-old woman with right upper lobe poorly differentiated carcinoma (c-T4N3M1c, stage IVB) diagnosed with MET exon 14 skipping mutation in July 2021, and she was given oral savatinib 200 mg in August 2021 25-day efficacy assessment as partial response (PR)
.
Another case was a liver transplant patient who was diagnosed with MET exon 14 skipping mutation of left upper lung adenocarcinoma (c-T3N3M1a, stage IV) in September 2021, and was given oral savatinib 200mg qd and gradually increased to 3 weeks.
600mg qd
.
Likewise, the Nov.
9, 2021 efficacy assessment was PR
.
Currently, a phase IIIb confirmatory clinical study (registration number: CTR20211151) of sevolitinib in the treatment of patients with locally advanced or metastatic NSCLC with MET exon 14 skipping mutation with Professor Lu Shun as the main PI is ongoing
.
This is a single-arm, open-label, multicenter phase III study with a planned enrollment of 163 patients divided into 2 cohorts
.
Cohort 1 included patients with disease progression or toxicity intolerance after previous platinum-containing chemotherapy regimens, and cohort 2 included patients who had not received any prior systemic antitumor drug therapy for advanced disease
.
The primary endpoint was IRC-assessed ORR
.
2.
MET amplification and overexpression: There is limited clinical treatment experience, and many explorations are initiating MET amplification as both a primary driver gene and a secondary/co-driver gene
.
MET amplification is one of the important mechanisms of EGFR-TKI resistance
.
Currently, treatment options for MET amplification-driven EGFR-TKI-resistant NSCLC are being explored
.
Amivantamab combined with lazertinib showed a trend of durable remission, and the safety was controllable; Capmatinib combined with gefitinib, Tepotinib combined with gefitinib showed good efficacy; savatinib opened the first combined osimertinib treatment of MET expansion A study of NSCLC (TATTON), and found that regardless of T790M status, savatinib + osimertinib has good and durable anti-tumor activity
.
Data from two expansion cohorts (B and D) of the TATTON study have been published
.
Among the 138 patients included in the Part B cohort, 69 patients had disease progression after receiving third-generation EGFR-TKI therapy (B1), 51 patients had not received third-generation EGFR-TKI therapy before and were T790M negative (B2), and 18 patients were previously Third-generation EGFR-TKI-naïve and T790M-positive (B3); Part D cohort included 42 previously third-generation EGFR-TKI-naïve and T790M-negative patients who received once-daily oral osimertinib + sivotinib combination therapy
.
The results showed that the ORR of parts B1, B2 and B3 were 33%, 65% and 67%, DCR was 75%, 88% and 100%, respectively, and the median PFS was 5.
5 months, 9.
1 months and 11.
1 months, respectively ; Part D ORR was 62%, DCR was 93%, and median PFS was 9.
0 months
.
Currently, more sevolitinib + osimertinib combination therapy options are being explored, including evaluating osimertinib combined with savatinib in the treatment of EGFRm and MET+ locally advanced or metastatic disease that has progressed after osimertinib treatment Phase II, Single-Arm SAVANNAH Study of Efficacy in Patients with NSCLC, Phase III SACHI Study of Cavatinib and Osimertinib Versus Pemetrexed and Platinum in NSCLC with MET Amplification Progressing After EGFR-TKI Therapy (China Registry Study), and Phase III SAFFRON Study (Global Registry Study) of savatinib combined with osimertinib versus chemotherapy in NSCLC with MET amplification/overexpression that has progressed after osimertinib treatment
.
Primary MET amplification, MET amplification and EGFR co-driving, and MET fusion currently have limited clinical treatment experience, and a number of explorations are being initiated, including sevolitinib combined with durvalumab in the treatment of EGFR wild-type with MET abnormalities in China The locally advanced or metastatic NSCLC study (SOUND), and the phase III study of savatinib plus osimertinib versus placebo plus osimertinib in the first-line treatment of EGFRm and MET overexpressing advanced NSCLC (SANOVO)
.
let us wait and see! Based on the present, looking to the future - MET targeted diagnosis and treatment experience sharing and hot spot discussions , and start a hot discussion
.
1.
Manage adverse reactions and optimize clinical decision-making In the first part of the discussion session, Professor Dong Xiaorong of Union Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Professor Jian Hong of Shanghai Jiaotong University Affiliated Chest Hospital, Professor Li Ziming of Shanghai Jiaotong University Affiliated Chest Hospital, Professor Yang Jinji from Guangdong Provincial People's Hospital and Professor Zhao Jun from Peking University Cancer Hospital had a heated discussion on the following three topics: What are the adverse reactions that you are more concerned about in the clinical application of savatinib? What is your experience with the management and dose adjustment of savatinib-related AEs? When encountering patients with other MET abnormalities (MET amplification/overexpression/fusion, etc.
) other than MET exon 14 skipping mutation, how would you consider the treatment options? Prof.
Zhao Jun, Prof.
Dong Xiaorong and Prof.
Yang Jinji▎Professor Zhao Jun: Edema and abnormal liver function are common toxicities during the clinical use of sevolitinib.
One patient developed allergic toxicity, including fever and rash.
It may also be less reported in the past
.
However, in other patients, the overall safety was good and generally controllable
.
Clinically, in addition to the detection of DNA, RNA-based detection is also a challenge, including the extraction of specimens
.
In addition, immunohistochemistry (IHC) is cheap and convenient in the detection of MET.
The detection and application of IHC for MET overexpression is also worthy of our attention in the future
.
▎Professor Dong Xiaorong: After the launch of savatinib, we have also applied it in the clinic for the three types of MET abnormalities (overexpression/amplification/exon 14 skipping mutation)
.
In terms of adverse reactions, for example, a 50-year-old patient developed high fever (39°C-40°C) and grade 1-2 edema after taking savatinib.
After symptomatic treatment, the fever recurred, and after drug withdrawal Symptoms disappeared
.
The patient's treatment effect achieved partial remission
.
Subsequently, we repeatedly adjusted the dose of sivotinib, but there was still some fever
.
Therefore, whether the cause of fever with savatinib is related to the patient's constitution still needs our attention and discussion
.
▎Professor Yang Jinji: For patients with EGFR mutations who are resistant to targeted therapy, MET fusion will also occur.
Whether the corresponding targeted therapy can be used remains to be determined
.
In addition, based on the patients with MET fusion after DNA-NGS detection, preliminary observations show that the efficacy of dual-target therapy in this population is limited
.
Whether it needs to be further verified by RNA-NGS remains to be further explored
.
Professor Jian Hong▎Professor Jianhong: At present, sevolitinib is mainly used in patients with MET14 exon skipping mutation, and fever is an adverse reaction worthy of attention
.
In addition, for patients with MET amplification after EGFR-TKI resistance, I think detection is very important and can be screened by FISH
.
For this population, sevolitinib 300 mg was well tolerated
.
Regarding MET overexpression, more research is needed to confirm the efficacy of targeted therapy, but the treatment options for these patients are limited, and targeted therapy may be a "breakthrough"
.
Professor Li Ziming▎Professor Li Ziming: For patients with MET exon 14 skipping mutation who developed abnormal liver function after taking 600mg of sevolitinib, reducing the dose of sevolitinib to 400mg, coupled with liver protection therapy, may make liver function worse back to normal
.
Most MET amplification or overexpression occurs after secondary drug resistance, which may be different from primary MET amplification or overexpression, and more research is needed to explore
.
▎Professor Lu Shun: For allergic reactions, such as fever and rash, we use hormonal drugs, and the symptoms are generally relieved
.
In addition, liver function damage is also worthy of our attention, especially when abnormal bilirubin and albumin indicators occur, reducing the dose of savatinib is a solution that can be considered
.
At present, the dose of sivotinib is mainly judged according to body weight in clinical practice, and experience and experience still need to be summarized in clinical practice in the future
.
2.
Precise treatment, detection first As a guiding speech, Professor Zhou Xiaoyan of Fudan University Affiliated Cancer Hospital first introduced the main points of clinical practice of MET detection
.
Among them, the commonly used detection methods for MET exon 14 skipping mutation are RT-qPCR and NGS, the commonly used detection methods for MET gene amplification are FISH and NGS, and the detection method for MET protein overexpression is IHC
.
Different detection methods have their own advantages and disadvantages, and the appropriate detection method should be selected according to the actual situation in clinical practice
.
Next, Professor Zhou Xiaoyan, Professor Ai Xinghao of Shanghai Jiaotong University Chest Hospital, Professor Fan Min of Fudan University Cancer Hospital, Professor Gao Beili of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Professor Han Yuchen of Shanghai Jiaotong University Chest Hospital, Fudan University Affiliated Professor Hu Jie of Zhongshan Hospital and Professor Wang Jialei of Fudan University Affiliated Cancer Hospital discussed the following questions: How to accurately detect MET14 exon skipping mutation? Do you think RT-qPCR is reliable for detecting MET14 exon skipping mutations? RNA-based assays have higher detection capabilities, but RNA samples are easily degraded.
How to efficiently achieve tissue extraction from patients? May I ask what kind of patients are considered clinically for MET amplification testing? What testing method will be chosen? Which method do you think needs improvement to better detect MET gene amplification? At present, there is no unified standard for the cutoff value of different MET amplification detection methods.
How do you consider the timing of starting treatment? At what threshold is MET protein overexpression clinically significant? Whether the clinic will consider initiating treatment
.
Professor Ai Xinghao▎Professor Ai Xinghao: Clinically, the incidence of MET amplification detected in EGFR-TKI-resistant patients does not reach the incidence reported in the literature
.
In addition, there are still doubts about the correlation of MET amplification, FISH and NGS detection results, and the effect of local amplification and multiligand amplification on the efficacy of MET-TKI
.
Professor Han Yuchen▎Professor Han Yuchen: For non-small cell lung cancer, we emphasize multi-gene testing
.
For patients with MET amplifications that are not abundant after NGS detection, FISH verification may be necessary
.
In terms of optimizing the process, we look forward to the release of relevant consensus
.
Data is still needed to determine whether immunohistochemistry can be used as MET amplification
.
Professor Fan Min▎Professor Fan Min: RNA-NGS is often used in the inspection of MET in our hospital, but it is highly dependent on the platform
.
In the past, MET overexpression was considered to be less able to guide clinical practice, and the criteria for interpreting MET amplification still need to be clarified
.
Prof.
Beili Gao ▎ Prof.
Beili Gao: At present, clinically, it is still difficult to start MET-TKI therapy in patients with MET overexpression
.
In addition, whether immunohistochemistry can be directly qualitative or not must be verified by NGS, and how to optimize the treatment plan with existing treatment methods is also a clinical problem that needs to be solved
.
Prof.
Hu Jie ▎ Prof.
Hu Jie: For the MET signaling pathway, the MET exon 14 skipping mutation has been well defined, but the MET amplification is still controversial
.
For example, DNA-level MET amplification detection includes FISH and NGS, but the detection results of different methods are different.
Will this affect the treatment effect? As a clinician, I want patients to receive precise care without over-testing and under-treatment
.
Professor Wang Jialei▎Professor Wang Jialei: The situation of abnormal MET pathway is very complicated.
In terms of treatment, how to optimize the treatment sequence and how to balance the efficacy and safety in clinical practice; in terms of detection, how to solve the problems of tumor heterogeneity and cross-mutation, etc.
resolve
.
Summary: Professor Liao Meilin concluded at the end that this meeting brought together pathology departments and clinicians, and promoted exchanges and learning between the two departments
.
In addition, as a big country in lung cancer, China is in urgent need of its own innovative drugs.
It is hoped that more innovative drugs like savatinib will be developed and marketed in the future to provide more clinical solutions
.
*This article is only used to provide scientific information to medical professionals and does not represent the views of this platform