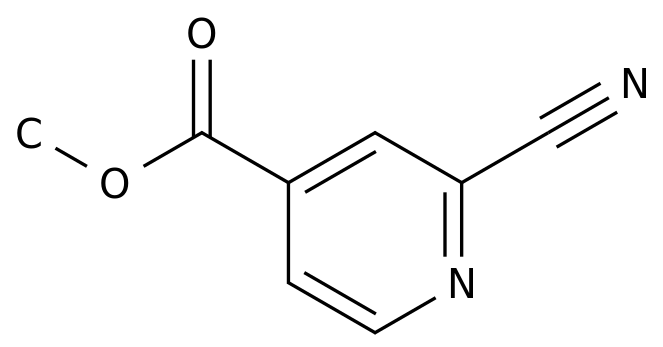
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Global biotechnology breakthrough and rapid evolution of industrialization, technology turnover and the results of the transformation cycle is shortening, cell therapy as the representative of the new technology has become an important driving force in recent years in the development of the biopharmaceutical industry, is expected to become the "third pillar of medicine."
According to the principle of treatment, cell therapy is mainly divided into stem cell therapy (mainly used in rare diseases, major chronic diseases and severe trauma repair in the field of regenerative medicine) and immunocell therapy (mainly used in the field of tumors), gradually become an indispensable and effective means to make up for traditional treatment.
the new crown pneumonia as an example, the current global stem cell treatment of new crown pneumonia research projects are in full swing.
Wuhan has completed more than 200 cases of stem cell therapy, the United States, South Korea, the United Arab Emirates, etc. have completed some of the new crown/severe patient stem cell therapy, clinical treatment safety is good, for the new crown pneumonia treatment provides a new method and model.
42 cell therapy products are currently available worldwide, and about 7,900 clinical trials of cell therapy products have been registered.
although there are still many engineering and regulatory challenges in cell therapy, with the breakthrough of basic research and the rapid development of clinical applications, the cell industry has caused huge market space.
the stem cell industry is expected to be about $400 billion globally by 2020, and immunocellular therapy will account for at least 10 percent of the total tumor treatment market.
The development of cell industry is no longer a choice to seize the commanding heights of biotechnology and biological industry has become a major strategy for countries to improve international competitiveness and master the initiative to develop, cell industry as an important area of a new round of global biotechnology changes, as a key direction to support development.
For example, to encourage car-T cell therapy-based immunotherapy, the U.S. Congress has allocated $1.8 billion for the Cancer Moon Project, and Japan and the United Kingdom have introduced a national system of stem cell therapy to encourage and promote the development of a well-regulated cell industry.
Although the development of the cell industry is faced with many quality testing standards and regulatory norms, the Wei Tracy incident in 2016 caused China's cell industry to stagnate for some time, but the country has issued "cell therapy product research and evaluation technical guidelines (trial)," biomedical new technology clinical application management regulations (draft for comments), somatic cell therapy clinical research and conversion application management methods (trial draft) (trial draft) and so on, for the domestic cell therapy industry to develop detailed general and general guidelines.
At the same time, China has also clearly formulated a series of guidelines and policies to accelerate the development of the cell industry, the "13th Five-Year Plan" National Strategic Emerging Industries Development Plan, the Strategic Emerging Industries Key Products and Services Guidance Directory, the "13th Five-Year Plan" bio-industry development plan, the "13th Five-Year Plan" special plan for health and health science and technology innovation, the "Action Plan for the Promotion of High-Quality Development of the Health Industry (2019-2022)" and other key technical research to strengthen stem cells, immunocellular therapy and so on.
the 2019 edition of the Industrial Structure Adjustment Guidance Directory, which is regarded as the trend of industry development, adds cell therapy drugs to the encouragement category for the first time, marking that cell therapy in China has entered the stage of industrialization.
2019 alone, 11 local governments and industrial parks have introduced support policies to explicitly support the development of the cell industry.
, the development of the cell industry not only responds positively to the national call, but also opens up the opportunity of regional industrial competitiveness oversized, which has a profound impact on the changes in the pattern of the biopharmaceutical industry.
the post-epidemic era around the layout of biomedicine, the concept of the cell industry is hot, not talking about "whether to develop the cell industry", but "how to develop the cell industry."
the development of cell industry in local governments or parks needs professional systematic layout compared with other sub-sectors of biomedicine, local governments or parks to develop cell industry has a certain speciality.
, the development of cell industry is still in its infancy, cell therapy has not yet formed a corresponding industrial cluster, domestic and foreign can learn from the development experience is very small.
Second, cell therapy is a technology-intensive emerging technology, with a large number of speculative and profit-based clinical studies, high potential risks, the success rate of preclinical projects is generally very low, most products at least until the clinical II. period will be clear its potential value, in the short term it is difficult to achieve economic benefits of the industry.
professional and accurate assessment is required for the introduction of screening of related enterprises or projects.
, China's access requirements for the cell industry are very high.
such as the Special Management Measures for Foreign Investment Access (Negative List) (2020 Edition) clearly stipulates that foreign investment in "the development and application of human stem cells, genetic diagnosis and therapeutic technologies" is prohibited.
barriers and investment mine zones in different industrial chains of the cell industry need to be carefully screened.
, the industrial chain of the cell industry is extremely long, including cell extraction and storage, quality control testing, research and development and production, clinical applications, cold chain logistics, etc., but the development of different links is difficult.
Local governments or parks need to combine the industrial base of different industrial links such as research institutes, existing enterprises, clinical filing institutions for stem cells or immune cells, etc., to compare the development prospects, market value, entry threshold and difficulty, competitiveness, major enterprises and projects, etc., to clarify the layout priority of the cell industry development system.
This five, cell therapy in recent years has only made rapid progress, most of them are start-up or growth stage of small and medium-sized innovative enterprises, is in the important stage of breeding to be developed, only by their own to achieve rapid development is very difficult, in addition to financial support, but also for the cell industry public service platform, GMP plant and other related supporting support.
local governments or parks need to professionally design targeted and truly service-oriented technical service platforms and support systems to attract outstanding enterprises and projects.
six, enterprises stationed in the local or park is most concerned about one is supporting, the other is talent, cell therapy as the cutting-edge biotechnology on the talent requirements are very high.
importance of introducing research and development talents is self-evident, but the particularity of the cell industry lies in the extreme shortage of talents such as cell screening, cell plant construction optimization, quality control testing, etc.
choose which talents, how to retain the need to be based on the layout of the cell industry system to organize the pulse.
Visible cell industry", "where the development path" is by no means a hot-headed on the hot development, but the need to combine national policy guidance, industrial development trends and regional competition patterns, in the analysis of the industrial base, tap potential, analysis of pain points on the basis of clear target positioning and development path.
professional system planning layout for the development of the cell industry height and speed, the introduction of talent, project landing is very important.