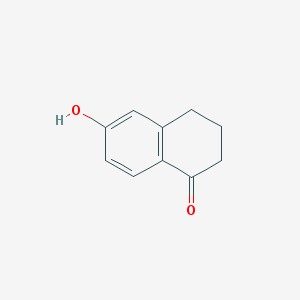
The complete remission rate of imfinzi + lynparza is 50%
-
Last Update: 2020-02-17
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
Recently, ASCO-GU was held in San Francisco A single arm phase II study (nct03534492) published on 2020 showed that the combination of AstraZeneca's anti PD-L1 therapy, imfinzi (yingfeifan, general name: durvalumab, duvalizumab), and targeted anticancer drug, lynparza (general name: olaparib, olapari), showed strong efficacy in the new adjuvant treatment of muscle invasive bladder cancer (MIBC): disease The complete physical remission rate (PCR) reached 50% In this study, 29 patients with ct2-t4a resectable MIBC were enrolled These patients were treated with imfinzi 6-8 weeks before planned cystectomy, 1500 mg once every 4 weeks for 2 months (maximum 2 doses / cycle), and lynparza twice a day for 150 mg twice a day for 56 days (28 days, 2 cycles) Twenty patients who received cystectomy were evaluated for efficacy and 27 for safety In terms of ECoG physical state score, 55.2% of the patients in this study were 0 (the activity ability was completely normal, no significant difference from before the onset of the disease), 44.8% of the patients were 1 (able to walk and engage in light physical activity, but not engage in heavy physical activity) The median age of the patients was 71 years old In TNM stage, pT2 accounted for 82.2%, pT3 for 7.1% and pT4 for 7.1% About 14.3% of the patients had lymph node spread The results showed that 10 out of 20 patients (50%) received complete pathological remission (PCR), which was defined as no tumor in bladder and lymph node In the safety assessment, The combination of imfinzi and lynparza was well tolerated Among the 27 patients who evaluated the safety, only 3 cases of grade 4 adverse events (one case for wound enucleation, one case for bleeding, one case for septic shock, respectively) and 3 cases of treatment interruption (due to personal decision, progressive disease, deterioration of chronic obstructive pulmonary disease) occurred At present, researchers are analyzing the expression of PD-L1 and the pattern of immune infiltration in blood samples before and after treatment All tumor samples were sequenced Juan Francisco Rodriguez Moreno, MD, oncologist of the comprehensive cancer center of CICC, HM hospital, Madrid, Spain, who reported the study, said: "this study is not intended to assess the efficacy of these two drugs With this in mind, the data on efficacy are interesting because 50% of the PCR rate is superior to other therapies in new adjuvant therapy The main purpose of this study is to determine the impact of this combination on molecular characteristics, but biomarker data are currently being analyzed and are expected to be published above ESMO 2020 in Madrid " Rodriguez Moreno said that the standard care for muscle infiltrating bladder cancer (MIBC) is radical cystectomy, which is rarely cured Although neoadjuvant chemotherapy based on cisplatin is the standard perioperative care, only a few patients can benefit from it Therefore, new adjuvant therapy is needed Recent evidence suggests that immunocheckpoint inhibitors can be incorporated into this therapeutic environment, and that they have been approved by the US FDA for the treatment of patients with advanced bladder cancer who are difficult to treat or unqualified for platinum chemotherapy However, the highest response rate of MIBC patients to immunosuppressive monotherapy was about 30% Imfinzi has been shown to be effective in patients with advanced urothelial carcinoma (UC) who have received chemotherapy with platinum, and is currently being evaluated for first-line treatment, including monotherapy and combination therapy with tremelimumab, an anti-CTLA-4 therapy At the ASCO meeting in 2019, the preliminary results of the single arm trial of imfinzi + tremelimumab combined therapy for high-risk MIBC patients who did not meet the neoadjuvant chemotherapy conditions of cisplatin showed that in 21 patients who had undergone complete cystectomy, the PCR rate of the combined therapy was 43% (9 / 21), and in 14 patients (67%) the tumor stage decreased.
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.