-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Basic condition of the patient
Gender: Female
Age: 57 years old
Basic information: In March 2015, the patient underwent thyroidectomy, etc.
, and received the last radioactive iodine treatment
on May 18, 2016.
In order to monitor the progression of the disease, the patient underwent imaging tests in 2018 and 2019, and the last imaging results showed multiple nodules in both lungs, multiple small lymph nodes in the mediastinum, and disease progression
.
Personal history: no history of smoking, alcohol consumption, allergies, or substance abuse
The patient has been treated previously
On March 6, 2015, the patient underwent thyroidectomy, etc
Surgical treatment: thyroidectomy + cervical lymph node dissection + lymphadenectomy + peripheral nerve surgery + bronchotomy;
Pathological result: papillary thyroid carcinoma (PTC), grade unknown
.
From 13 June 2015 to 18 May 2016, the patient was treated with radioactive iodine
Treatment under:
On 13 June 2015, the patient was treated with radioactive iodine at 100 mCi;
On 26 November 2015, the patient received 150mCi radioactive iodine therapy;
On 18 May 2016, the patient was treated
with radioactive iodine at 150 mCi.
On May 22, 2016, treated ¹³¹I systemic tomographic imaging showed that the lesions did not take iodine
.
Disease progression was detected at patient review on 21 March 2019
After imaging evaluation, the right lower lung nodule is 11mm, multiple nodules in both lungs, multiple small lymph nodes in the mediastinum, and the patient has disease progression (PD).
After screening, the patient met the "multicenter, randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of donafenib tosylate tablets in the treatment of locally advanced/metastatic iodine-refractory differentiated thyroid cancer (RAIR-DTC)" (ZGDD3 study) conducted by Henan Cancer Hospital and was enrolled in the clinical trial
.
Donafenib treatment undergoes
Patient baseline: height 160 cm, weight 69 kg, BMI 26.
95 kg/m², Eastern Collaborative Oncology Group physical status score (ECOG PS) 1, no previous history of
tyrosine kinase inhibitor (TKI) therapy.Tumor baseline: T4aN1bM1 (lung/lymph node metastasis), target lesion 11mm in the right lower lung, multiple nodules in both lungs, and multiple small lymph nodes
in the mediastinum.Laboratory baseline: thyroid-stimulating hormone (TSH) 0.
07 mIU/L (local laboratory), thyroglobulin (Tg) 40.
1 ng/mL, thyroglobulin antibody (TgAb) 0.
1 IU/mL (local laboratory).On 3 April 2019, patients were enrolled in the trial and started oral multikinase inhibitor donafenib (300 mg twice daily).
At week 8 of enrollment, the patient's Tg level decreased to 10.
14 ng/ml, the target lesion was reduced by 45.
5% from baseline, and the disease was partially relieved (PR).At week 24, the patient had a minimum SOD of target lesions and retreated to 5 mm
.The patient is still receiving donafenib (41 months old).
Laboratory evaluation
Tg levels and target lesion size changed
from the screening period to 144 weeks of treatment.
Imaging tests
From baseline to September 17, 2019, radiographic changes
in lung target lesions.
From baseline to 17 September 2019, radiographic changes
in non-target lesions in the lungs.
Donafenib treatment-related adverse reactions (TRAE) occur
Other TRAEs are CTCAE grade 1 and include diarrhea, arthralgias, rash, ectopic rhythm, atrial escape rhythm, and elevated
phosphate.
The patient's ECOG PS during treatment dropped to 0 on day 28 and remained until the end of the
trial.
Case summary
This case was diagnosed with PTC, and after surgical treatment such as thyroidectomy, the disease progressed after multiple radioactive iodine treatments, and lung/lymph node metastases
occurred.
By ¹³¹I systemic tomographic imaging, the patient was diagnosed that the disease had progressed to iodine refractory
.
On April 3, 2019, after multiple evaluations, the patient met the eligibility criteria for the ZGDD3 study and was enrolled in the clinical study
.
At the first efficacy assessment, donafenib showed good efficacy: Tg levels decreased significantly at week 4 of enrollment and remained stable during treatment
.
At the 8th week of treatment, imaging findings suggest that the tumor has reached PR.
At week 24 of treatment, the tumor reaches a minimum SOD (5 mm).
So far, the patient is still taking donafenib monotherapy, and his progression-free survival (PFS) has been nearly 41 months, and the tumor is in PR state
.
During treatment, patients did not develop grade 3 TRAEs and donafenib was generally well
tolerated.
Expert reviews
Professor Yang Hui
Chief physician, master tutor and department director of the Department of Nuclear Medicine, Henan Cancer Hospital
Director of Henan Provincial Key Laboratory of Precision Diagnosis and Treatment of Nuclear Oncology
He is the chairman-elect of the Tumor Nuclear Medicine Professional Committee of the Chinese Anti-Cancer Association
Vice Chairman of the Nuclear Medicine Expert Committee of the Chinese Society of Clinical Oncology
Member of the Standing Committee of the Nuclear Medicine Branch of the Chinese Society of Imaging Technology
Vice Chairman of the Radioactive Particle Working Committee of the Nuclear Medicine Branch of the Chinese Medical Association
Vice Chairman of the Particle Therapy Branch of the Minimally Invasive Tumor Treatment Professional Committee of the Chinese Anti-Cancer Association
Member of the Standing Committee of the Editorial Board of the Journal of Tumor Imaging
As the most common endocrine malignancy, the incidence of thyroid cancer has continued to increase in recent years, of which PTC is the most common pathological type, often referred to as differentiated thyroid cancer (DTC) together with follicular thyroid carcinoma (FTC) [1].
The vast majority of patients have a good prognosis and long-term survival after standardized treatment, but some patients still develop distant metastases after treatment and lose the ability to concentrate iodine during natural history or treatment, and develop iodine-refractory differentiated thyroid cancer (RAIR-DTC) [2].
Compared with DTC patients with good iodine intake, patients with RAIR-DTC generally have shorter survivals, with an average survival of only about 3 to 5 years and a 10-year survival rate of about 10 percent [3].
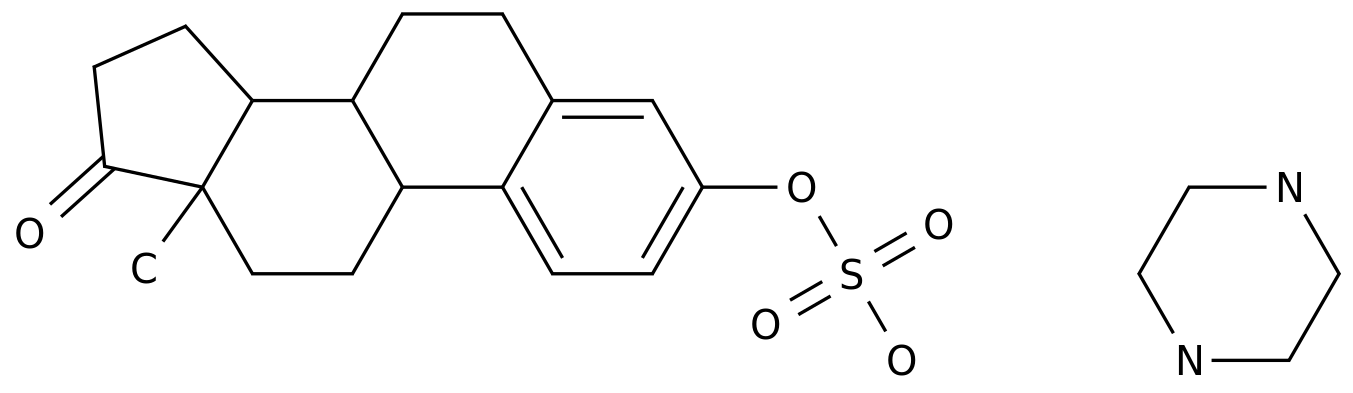
Donafenib is a novel oral multikinase inhibitor, which is a new patented drug molecule formed by substituting pyridylmethanamine on the sorafenib molecule
with pyridyltrideuterated methylamine.
At present, pharmacodynamics, pharmacokinetics (PK), clinical efficacy and safety data have proved that compared with sorafenib, donafenib has unchanged antitumor activity in vitro, in vivo antitumor activity is enhanced and the safety is improved [4].
。 This week, Donafenib's DIRECTION study, presented at the 2022 American Thyroid Association (ATA) Annual Meeting, is a randomized, double-blind, placebo-controlled, multicenter phase III study that currently includes the largest number of RAIR-DTC patients in China, to evaluate the efficacy and safety
of donafenib in Chinese RAIR-DTC patients.
The study included 191 patients with locally advanced/metastatic RAIR-DTC and randomly divided them into donafenib (n=128) and placebo (n=63)
in a 2:1 ratio.
The results of the study showed that donafenib significantly prolonged the independent review committee (IRC) assessed mPFS (12.
9 versus 6.
4 months, HR=0.
39, p<0.
0001)<b15> compared with placebo.
In terms of objective response rate (ORR), the donafenib group was significantly higher than the placebo group (23.
3 percent vs.
1.
7 percent) [5].
In this case, after nearly 41 months of first-line treatment with donafenib, the patient has not developed acquired resistance and the disease remains in PR status
.
It is believed that donafenib is expected to become the treatment of RAIR-DTC patients in China in the future
.
References
[1] CHEN Wenjie, WANG Yabing, CHEN Xiaolin, et al.
Recent research progress of iodine-refractory differentiated thyroid cancer[J].
Chinese Journal of Clinical Pharmacology and Therapeutics,2022,27(01):116-120.
)[2] Wang Renfei, Wang Yong, Shi Feng, et al.
Consensus on the diagnosis and treatment of iodine-refractory differentiated thyroid cancer (2019 edition).
China Oncology,2019,29(06):476-480.
)[3] LIU Yanqing,LIN Yansong.
Diagnosis and treatment strategy and prognosis of iodine-refractory differentiated thyroid cancer[J].
Chinese Journal of Practical Surgery,2019,39(03):216-220.
DOI:10.
19538/j.
cjps.
issn1005-2208.
2019.
03.
07.[4] Li Su, Qin Shukui.
Deuterated drugs and their application prospects in the research and development of new antitumor drugs[J].
Journal of Clinical Oncology,2020,25(12):1138-1143.
)[5] Y.
Lin, H.
Yang, F.
Shi, et al.
Donafenib in Locally Advanced/Metastatic, Radioactive Iodine-Refractory, Differentiated Thyroid Cancer: A Randomized, Double-blind, Placebo-controlled, Multi-Center Phase III Clinical Trial (DIRECTION).
ESMO Abstract 3099.
Typesetting: Youshi
Execution: Small garden
This platform aims to deliver more medical information
to healthcare professionals.
The content published on this platform cannot replace professional medical guidance in any way, nor should it be regarded as diagnosis and treatment advice
.
If such information is used for purposes other than understanding medical information, this platform does not assume relevant responsibilities
.
This Pingtai does not agree with the description and views
of the content of this posting.
If copyright issues are involved, please contact us and we will deal with it
as soon as possible.