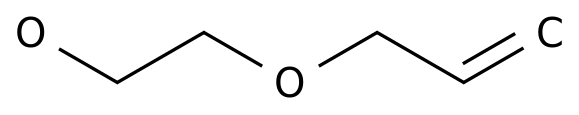
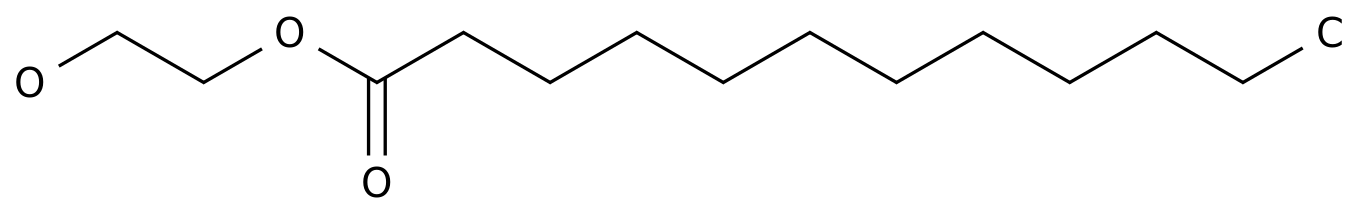
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
[ Focus on Chemical Machinery Equipment Network ] Under the implementation of policies such as consistency evaluation and the new version of GMP, in order to meet higher production requirements, pharmaceutical companies have soared demand for pharmaceutical equipment , which has driven the growth of the annual output value of the entire pharmaceutical equipment industry.
Data shows that during the five years from 2011 to 2015, the annual output value of my country's pharmaceutical equipment industry increased from 8.
96 billion yuan in 2011 to 20.
19 billion yuan in 2015, breaking the 20 billion mark, and the 5-year compound growth rate reached 34.
%.
Chemical machinery and equipment network hot attention to chemical machinery and pharmaceutical equipmentData shows that during the five years from 2011 to 2015, the annual output value of my country's pharmaceutical equipment industry increased from 8.
96 billion yuan in 2011 to 20.
19 billion yuan in 2015, breaking the 20 billion mark, and the 5-year compound growth rate reached 34.
%.
Today, the consistency evaluation policy is still advancing, and whether or not the over-evaluation affects whether pharmaceutical companies can participate in the national centralized drug procurement, to a certain extent, has become a market entry ticket for pharmaceutical companies.
Centralized procurement of drugs is one of the major policies that have affected the pharmaceutical industry in recent years.
Its purpose is to squeeze out the price of products through the "price-for-quantity" approach, and reduce the burden of treatment costs for patients.
At present, National Procurement has reached the fourth batch, of which the results of the fourth batch of centralized procurement were officially announced recently.
55 varieties were selected, with an average price reduction of 52%, involving 118 pharmaceutical companies to be selected.
Centralized procurement of drugs is one of the major policies that have affected the pharmaceutical industry in recent years.
Its purpose is to squeeze out the price of products through the "price-for-quantity" approach, and reduce the burden of treatment costs for patients.
At present, National Procurement has reached the fourth batch, of which the results of the fourth batch of centralized procurement were officially announced recently.
55 varieties were selected, with an average price reduction of 52%, involving 118 pharmaceutical companies to be selected.
Before the results of the fourth batch of centralized procurement were released, my country’s "Opinions on Promoting the Normalization and Institutionalization of Drug Centralized and Volume Procurement Work" clarified the normalization mechanism for centralized drug procurement.
The document also mentioned that it would explore the appropriate indications.
Or, different generic drugs with similar functions and indications will be merged to carry out centralized purchasing.
The document also mentioned that it would explore the appropriate indications.
Or, different generic drugs with similar functions and indications will be merged to carry out centralized purchasing.
The industry generally believes that the normalization of centralized procurement has become a trend, and more varieties including Chinese patent medicines and biological drugs will be included in centralized procurement in the future, driving more pharmaceutical companies to join the centralized procurement competition.
With the normalization of centralized procurement, pharmaceutical companies will accelerate the process of evaluating the consistency of generic drugs in order to obtain the qualifications to participate in centralized procurement.
In this context, pharmaceutical companies will also expand their demand for pharmaceutical equipment, bringing more room for development to the pharmaceutical equipment market.
With the normalization of centralized procurement, pharmaceutical companies will accelerate the process of evaluating the consistency of generic drugs in order to obtain the qualifications to participate in centralized procurement.
In this context, pharmaceutical companies will also expand their demand for pharmaceutical equipment, bringing more room for development to the pharmaceutical equipment market.
In fact, the policy side also supports encouraging the development of pharmaceutical equipment in the direction of transformation.
For example, in September 2020, * and other four departments jointly issued the "Guiding Opinions on Expanding Investment in Strategic Emerging Industries, Cultivating and Growing New Growth Pole Growth Pole", which proposed that the equipment manufacturing industry should be accelerated to make up for shortcomings.
The key projects supported include the production of medical equipment and pharmaceutical equipment, and the implementation of smart manufacturing and smart construction pilot demonstrations.
For example, in September 2020, * and other four departments jointly issued the "Guiding Opinions on Expanding Investment in Strategic Emerging Industries, Cultivating and Growing New Growth Pole Growth Pole", which proposed that the equipment manufacturing industry should be accelerated to make up for shortcomings.
The key projects supported include the production of medical equipment and pharmaceutical equipment, and the implementation of smart manufacturing and smart construction pilot demonstrations.
Aiming at the market, some large pharmaceutical companies are making efforts.
For example, in 2020, Xinma Pharmaceutical has promoted the pharmaceutical equipment industrialization project.
The launch of this project marks the further upgrade of Xinma Pharmaceutical’s technological innovation.
The company hopes to use the construction of this project to improve the level and competitiveness of the domestic pharmaceutical equipment industry and solve domestic problems.
The pain point of the pharmaceutical equipment industry; Canaan Technology announced the establishment of a wholly-owned subsidiary in May 2020.
On the same day, it also announced that it plans to participate in the bidding for the right to use the state-owned construction land of Yongjia County Gong 202006 for no more than 55 million yuan.
Relying on this industrial base, the company stated that it will continue to strengthen the core competitiveness of the three major business segments of the generic solid preparation smart factory business, biological preparation innovative medicinal water equipment and pharmaceutical liquid dispensing system engineering business; establish smart pharmaceuticals that meet the needs of the domestic market The research and manufacturing capabilities of equipment and intelligent logistics systems form a more complete industrial chain and further expand the company's business scale.
For example, in 2020, Xinma Pharmaceutical has promoted the pharmaceutical equipment industrialization project.
The launch of this project marks the further upgrade of Xinma Pharmaceutical’s technological innovation.
The company hopes to use the construction of this project to improve the level and competitiveness of the domestic pharmaceutical equipment industry and solve domestic problems.
The pain point of the pharmaceutical equipment industry; Canaan Technology announced the establishment of a wholly-owned subsidiary in May 2020.
On the same day, it also announced that it plans to participate in the bidding for the right to use the state-owned construction land of Yongjia County Gong 202006 for no more than 55 million yuan.
Relying on this industrial base, the company stated that it will continue to strengthen the core competitiveness of the three major business segments of the generic solid preparation smart factory business, biological preparation innovative medicinal water equipment and pharmaceutical liquid dispensing system engineering business; establish smart pharmaceuticals that meet the needs of the domestic market The research and manufacturing capabilities of equipment and intelligent logistics systems form a more complete industrial chain and further expand the company's business scale.
However, from the current overall situation, China's pharmaceutical equipment industry still has the problems of insufficient equipment and lack of high-tech pharmaceutical equipment manufacturers.
Some equipment such as centrifuges are mainly monopolized by imported products.
Insiders pointed out that the next ten years will be a golden period for the development of the pharmaceutical equipment industry, and the research and development of pharmaceutical equipment will become a new support for the continued growth of the entire industry in the future.
It can be seen that the transformation and upgrading of the domestic pharmaceutical machinery industry is imminent.
In recent years, domestic pharmaceutical machinery companies have gradually realized these problems.
The industry is moving towards rationality, science, and innovation.
The requirements for production quality and stability are also getting higher and higher.
Pharmaceutical equipment is accelerating towards integration, continuity, automation, and Tackling key issues such as informatization and intelligence.
Centrifuge Some equipment such as centrifuges are mainly monopolized by imported products.
Insiders pointed out that the next ten years will be a golden period for the development of the pharmaceutical equipment industry, and the research and development of pharmaceutical equipment will become a new support for the continued growth of the entire industry in the future.
It can be seen that the transformation and upgrading of the domestic pharmaceutical machinery industry is imminent.
In recent years, domestic pharmaceutical machinery companies have gradually realized these problems.
The industry is moving towards rationality, science, and innovation.
The requirements for production quality and stability are also getting higher and higher.
Pharmaceutical equipment is accelerating towards integration, continuity, automation, and Tackling key issues such as informatization and intelligence.
Original title: The "entry ticket" for over-rated pharmaceutical companies to participate in centralized procurement will drive the expansion of demand for pharmaceutical equipment