-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Hydrogen is a sustainable and clean energy that does not emit toxic gases and can add value to many industries such as transportation, power generation, and metal manufacturing
.
The technology of storing and transporting hydrogen bridges the gap between sustainable energy production and fuel usage.
More efficient hydrogen delivery systems can bring many benefits to applications such as fixed power sources, mobile power sources, and mobile cars, and therefore become a hydrogen economy.
An indispensable part of
.
However, traditional hydrogen storage and transportation technologies are very expensive, and hydrogen is easily contaminated
.
Therefore, researchers are always looking for reliable, low-cost and simple alternative technologies
.
According to foreign media reports, a team of researchers at the Lawrence Berkeley National Laboratory in the United States designed and synthesized an efficient material that can accelerate the extraction of hydrogen from alcohol
.
The material is a catalyst, composed of tiny clusters of nickel metal fixed on a 2D substrate
.
The research team found that this kind of catalyst can efficiently accelerate the reaction of removing hydrogen atoms from the liquid chemical carrier, and the material is very strong.
It is made of abundant metals instead of existing precious metals, which will help To make hydrogen an energy source for various applications
.
? Such compounds as catalysts are usually used to accelerate the rate of chemical reactions, and the compound itself is not consumed, but can keep a specific molecule in a stable position, or act as an intermediary to allow an important The chemical steps can be completed smoothly
.
As for the chemical reactions that can produce hydrogen from a liquid carrier, the most efficient catalysts are generally made of expensive metals
.
However, such catalysts are usually costly, have insufficient reserves, and are easily contaminated
.
The cheaper catalysts made of common metals tend to have low efficiency and poor stability, and their activity is limited, which also limits their practical application in the hydrogen production industry
.
? In order to improve this type of catalyst made of abundant metals, the researchers changed their strategy and focused on tiny, uniform nickel metal clusters
.
Such metal clusters are very important, allowing a certain amount of material to expose the active surface to the maximum
.
However, metal clusters are also easy to gather together, resulting in limited reaction capabilities
.
The researchers designed and conducted an experiment to prevent metal clusters from gathering together by depositing 1.
5 nanometer-sized nickel clusters on a 2D substrate
.
The 2D substrate is made of boron and nitrogen, and is designed into an atom-sized, grooved grid
.
The nickel clusters will be evenly and firmly fixed in the groove
.
This design can not only prevent metal clusters from gathering, but by directly interacting with nickel metal clusters, the thermochemical properties of the catalyst can also be greatly improved, thereby enhancing its overall performance
.
? The researchers used detailed X-ray and spectroscopic measurement results, combined with theoretical calculations, to reveal many conditions under the surface and the role of such conditions in the catalytic reaction
.
Berkeley Lab uses advanced photon source tools and computational modeling methods to identify changes in the physical and chemical properties of the 2D substrate when tiny nickel metal clusters are formed and deposited on the 2D substrate
.
The research team proposed that the material is formed when the metal clusters occupy the original area of the substrate and interact with the nearby edges, so that the small size of the metal clusters can be retained
.
Such tiny and stable metal clusters promote the separation of hydrogen from the liquid carrier, so that the catalyst has excellent separation properties, productivity and stability
.
? Calculations show that the size of the catalyst allows it to be more active than other best performing catalysts
.
Researchers used models and calculations to reveal the unique geometric and electronic structure of the tiny metal cluster
.
A large number of exposed metal atoms gather on such tiny clusters, and metal particles with larger sizes are more attractive to hydrogen carriers
.
Such exposed atoms can also slow down the step of hydrogen stripping from the carrier, while preventing the formation of contaminants that may clog the surface of the cluster
.
Therefore, the material can remain uncontaminated during the critical step of the hydrogen production reaction
.
Such catalysis and anti-pollution properties are deliberately introduced into the 2D substrate, which ultimately results in the clusters being able to maintain a small size
.
? In this study, the researchers succeeded in creating a cheaper, easily accessible, and stable material that can help strip hydrogen from the liquid carrier so that it can be used as a fuel
.
The research is based on the US Department of Energy's plan, which aims to study the requirements for hydrogen storage materials that can meet the Office of Energy Efficiency and Renewable Energy (EERE) Hydrogen and Fuel Cells, and optimize the materials to be used in vehicles in the future
.
? In the future, the Berkeley Lab team will further refine the strategy to change the 2D substrate so that it can support tiny metal clusters and develop more efficient catalysts
.
This technology helps to optimize the process of extracting hydrogen from liquid chemical carriers
.
.
The technology of storing and transporting hydrogen bridges the gap between sustainable energy production and fuel usage.
More efficient hydrogen delivery systems can bring many benefits to applications such as fixed power sources, mobile power sources, and mobile cars, and therefore become a hydrogen economy.
An indispensable part of
.
However, traditional hydrogen storage and transportation technologies are very expensive, and hydrogen is easily contaminated
.
Therefore, researchers are always looking for reliable, low-cost and simple alternative technologies
.
According to foreign media reports, a team of researchers at the Lawrence Berkeley National Laboratory in the United States designed and synthesized an efficient material that can accelerate the extraction of hydrogen from alcohol
.
The material is a catalyst, composed of tiny clusters of nickel metal fixed on a 2D substrate
.
The research team found that this kind of catalyst can efficiently accelerate the reaction of removing hydrogen atoms from the liquid chemical carrier, and the material is very strong.
It is made of abundant metals instead of existing precious metals, which will help To make hydrogen an energy source for various applications
.
? Such compounds as catalysts are usually used to accelerate the rate of chemical reactions, and the compound itself is not consumed, but can keep a specific molecule in a stable position, or act as an intermediary to allow an important The chemical steps can be completed smoothly
.
As for the chemical reactions that can produce hydrogen from a liquid carrier, the most efficient catalysts are generally made of expensive metals
.
However, such catalysts are usually costly, have insufficient reserves, and are easily contaminated
.
The cheaper catalysts made of common metals tend to have low efficiency and poor stability, and their activity is limited, which also limits their practical application in the hydrogen production industry
.
? In order to improve this type of catalyst made of abundant metals, the researchers changed their strategy and focused on tiny, uniform nickel metal clusters
.
Such metal clusters are very important, allowing a certain amount of material to expose the active surface to the maximum
.
However, metal clusters are also easy to gather together, resulting in limited reaction capabilities
.
The researchers designed and conducted an experiment to prevent metal clusters from gathering together by depositing 1.
5 nanometer-sized nickel clusters on a 2D substrate
.
The 2D substrate is made of boron and nitrogen, and is designed into an atom-sized, grooved grid
.
The nickel clusters will be evenly and firmly fixed in the groove
.
This design can not only prevent metal clusters from gathering, but by directly interacting with nickel metal clusters, the thermochemical properties of the catalyst can also be greatly improved, thereby enhancing its overall performance
.
? The researchers used detailed X-ray and spectroscopic measurement results, combined with theoretical calculations, to reveal many conditions under the surface and the role of such conditions in the catalytic reaction
.
Berkeley Lab uses advanced photon source tools and computational modeling methods to identify changes in the physical and chemical properties of the 2D substrate when tiny nickel metal clusters are formed and deposited on the 2D substrate
.
The research team proposed that the material is formed when the metal clusters occupy the original area of the substrate and interact with the nearby edges, so that the small size of the metal clusters can be retained
.
Such tiny and stable metal clusters promote the separation of hydrogen from the liquid carrier, so that the catalyst has excellent separation properties, productivity and stability
.
? Calculations show that the size of the catalyst allows it to be more active than other best performing catalysts
.
Researchers used models and calculations to reveal the unique geometric and electronic structure of the tiny metal cluster
.
A large number of exposed metal atoms gather on such tiny clusters, and metal particles with larger sizes are more attractive to hydrogen carriers
.
Such exposed atoms can also slow down the step of hydrogen stripping from the carrier, while preventing the formation of contaminants that may clog the surface of the cluster
.
Therefore, the material can remain uncontaminated during the critical step of the hydrogen production reaction
.
Such catalysis and anti-pollution properties are deliberately introduced into the 2D substrate, which ultimately results in the clusters being able to maintain a small size
.
? In this study, the researchers succeeded in creating a cheaper, easily accessible, and stable material that can help strip hydrogen from the liquid carrier so that it can be used as a fuel
.
The research is based on the US Department of Energy's plan, which aims to study the requirements for hydrogen storage materials that can meet the Office of Energy Efficiency and Renewable Energy (EERE) Hydrogen and Fuel Cells, and optimize the materials to be used in vehicles in the future
.
? In the future, the Berkeley Lab team will further refine the strategy to change the 2D substrate so that it can support tiny metal clusters and develop more efficient catalysts
.
This technology helps to optimize the process of extracting hydrogen from liquid chemical carriers
.




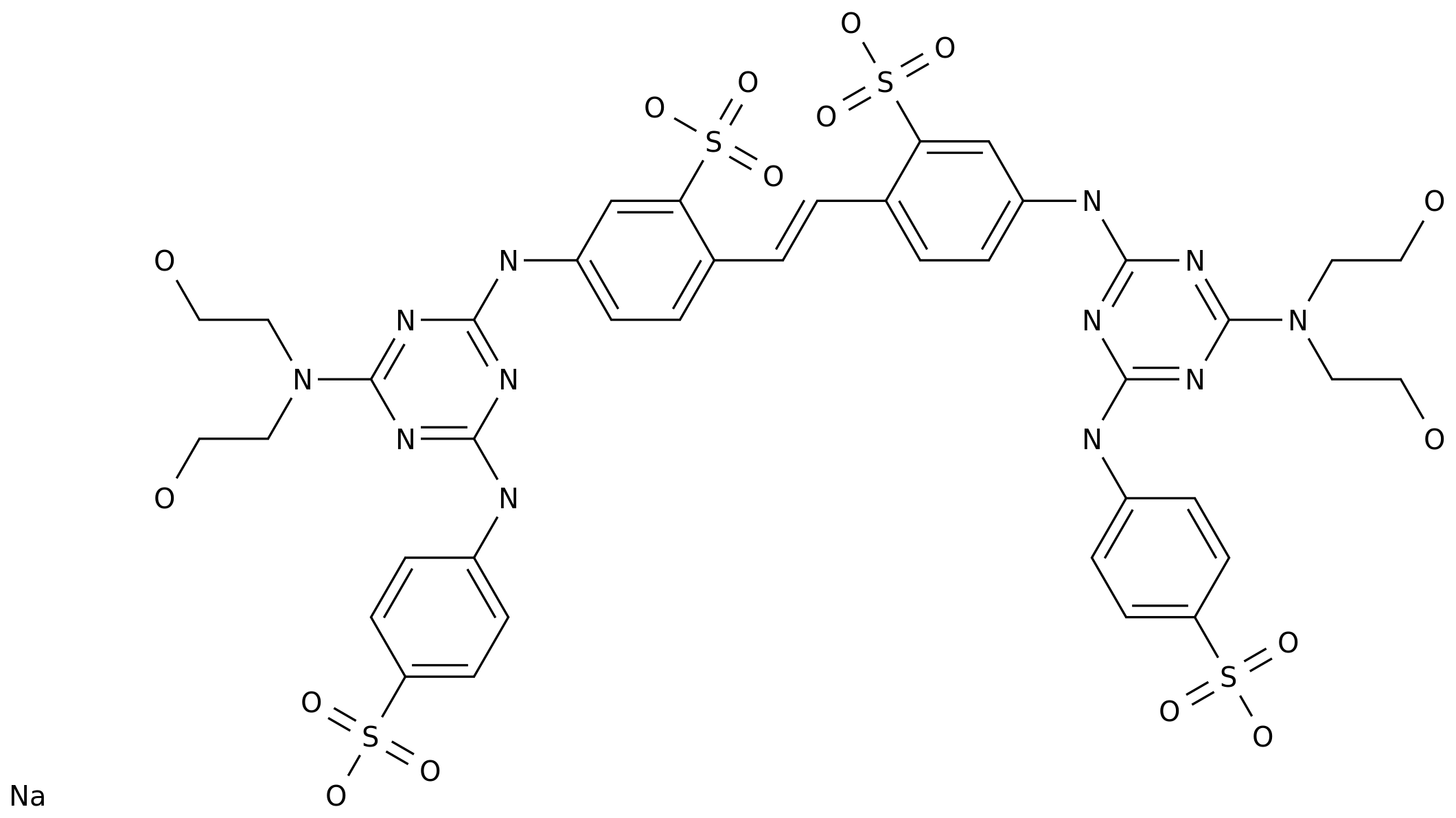
![disodium 4,4'-bis[[4-anilino-6-[(2-carbamoylethyl)(2-hydroxyethyl)amino]-1,3,5,-triazin-2-yl]amino]stilbene-2,2'-disulphonate CAS NO 27344-06-5](https://file.echemi.com/fileManage/upload/cas/77/e1abc71f-648d-403c-93fe-69b5c9401d56.gif)
