Uber reaches primary and secondary endpoints in the study of IL-17A/F single anti-drug bimekizuma in key Phase 3 trials
-
Last Update: 2020-06-07
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
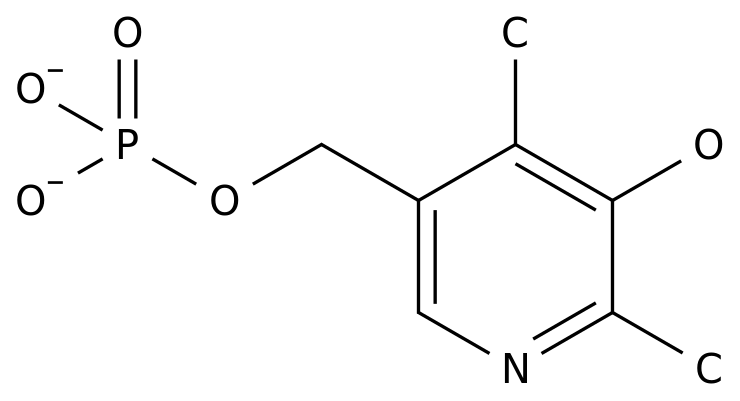
recently, UCB announced that bimekizumab,the(http:// of the substance IL-17A/F mono-anti-
drug for the treatment of chronic moderate to severe plaqueps (plaquepsis), had reached two common primary and all minor endpoints in the key phase 3trial(http://By mid-2020, Uber is expected to file a regulatory application for bimekizumabWhen the level imbalance between inflammatory cytokines and inhibition of inflammatory cytokines in the body can lead to the continued occurrence of chronic inflammatory diseases such as psoriasisBased on IL-12, IL-23/IL-17 signaling pathways play a key role in the release of pro-inflammatory cytokines, which has also become a popular target for the treatment of chronic inflammatory diseases by thePharmaceutical(http://(http://Full-humanized mono-anti-bimekizumab, capable of strong and specific allyification of IL-17A and IL-17FIL-17A plays a key role in the pathogenesis of plaque-like psoriasis, psoriasis arthritis and strong sylladesTHE IL-17A and IL-17F have more than 50% structural hoigenous and overlapping biological functionsIL-17A and IL-17F are raised in a variety of inflamed human tissues and act in synergy with other pro-inflammatory cytokines, such as tumor necrosis factors (TNf), to amplify the inflammatory responseCombining these two cytokines at the same time, preventing them from interacting with IL-17 receptors expressed on the surface of the cell, allowing bimekizumab to better perform anti-inflammatory functions A total of 435 patients participated in a 56-week BE READY trial designed to verify the effectiveness and safety of bimekizumab in treating chronic moderate to severe plaque-type psoriasis (diagnosed for more than 6 months, less than 10% of the affected area of the skin, PASI 12) compared to placebo The common primary endpoints of the trial were paSI 90 mitigation (at least 90% improvement in PASI scores for psoriasis area severity index) at week 16, and the overall assessment (IGA) score of 0 or 1 (removal or minimum disease) patient ratio Bimekizumab is statistically superior to placebo at all major and minor endpoints
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.