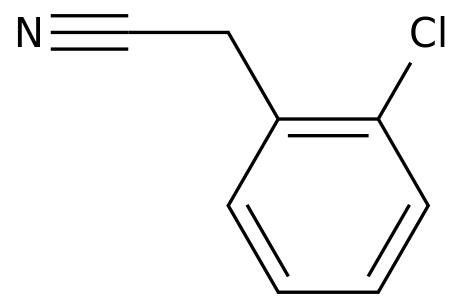
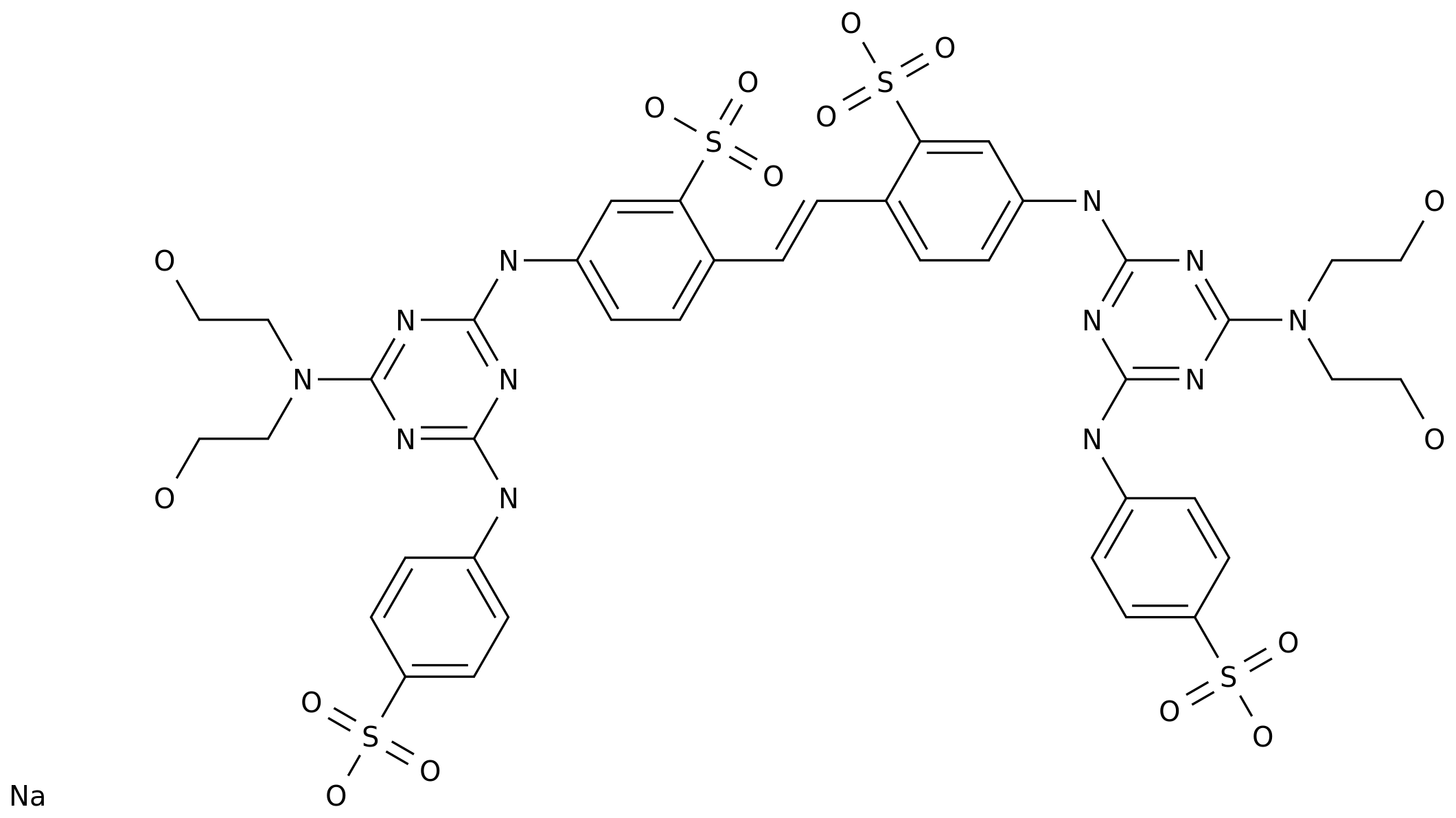
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Recently, the research group of Professor Gao Minrui of the University of Science and Technology of China used the "reaction-diffusion" mechanism to realize the Turing structure on inorganic transition metal chalcogenides for the first time.
Turing structures are widespread in nature, such as: zebra, sea fish, puffer fish, shell surface patterns, yellow sand ripples in the wind, fern leaves, sunflower phyllodes, and mouse hair follicle spacing and even hair density.
It is the result of "reaction-diffusion".
However, it is very difficult to construct a Turing structure in a chemical system, mainly because in most chemical systems, the difference in diffusion coefficients of substances is small, which does not meet the requirements of Turing structure formation, that is, the diffusion rate of activators is lower than that of inhibitors.
a lot of.
It was not until the 1990s that French scientists constructed a Turing pattern in the "chlorite-iodine-malonic acid reaction" based on the "reaction-diffusion" mechanism.
The researchers found that in the binary solution of diethylenetriamine (DETA) and water, Ag+ and DETA undergo a complex reaction to form Ag(DETA)+, while Co2+ overflows from the surface of the cobalt diselenide (CoSe2) nanobelt.
In this system, Ag(DETA)+ is the inhibitor and Co2+ is the activator.
When the rapidly diffused Ag(DETA)+ reaches the Nernst layer on the CoSe2 surface, it interacts with the activator Co2+ diffused to the CoSe2 surface, and finally forms a complex and beautiful Ag2Se Turing pattern on the CoSe2 surface.
Further research found that this multi-interface Turing structure Ag2Se-CoSe2 material is an efficient oxygen evolution electrocatalyst.
It only needs an overvoltage of 221mV to achieve an oxygen generation current density of 10mA/cm2, and its anode oxygen evolution efficiency is 84.
5%.
Experiments show that the OER activity of Ag2Se-CoSe2 is linearly related to the interface length of the Turing structure.
The rich interface structure and the optimized adsorption energy of the intermediate at the interface structure are the main reasons for its high activity.
This research uses the "reaction-diffusion" theory to build a complex Turing structure on inorganic nanostructured materials for the first time, providing new ideas for the design of cheaper catalysts with higher performance.
Turing structures are widespread in nature, such as: zebra, sea fish, puffer fish, shell surface patterns, yellow sand ripples in the wind, fern leaves, sunflower phyllodes, and mouse hair follicle spacing and even hair density.
It is the result of "reaction-diffusion".
However, it is very difficult to construct a Turing structure in a chemical system, mainly because in most chemical systems, the difference in diffusion coefficients of substances is small, which does not meet the requirements of Turing structure formation, that is, the diffusion rate of activators is lower than that of inhibitors.
a lot of.
It was not until the 1990s that French scientists constructed a Turing pattern in the "chlorite-iodine-malonic acid reaction" based on the "reaction-diffusion" mechanism.
The researchers found that in the binary solution of diethylenetriamine (DETA) and water, Ag+ and DETA undergo a complex reaction to form Ag(DETA)+, while Co2+ overflows from the surface of the cobalt diselenide (CoSe2) nanobelt.
In this system, Ag(DETA)+ is the inhibitor and Co2+ is the activator.
When the rapidly diffused Ag(DETA)+ reaches the Nernst layer on the CoSe2 surface, it interacts with the activator Co2+ diffused to the CoSe2 surface, and finally forms a complex and beautiful Ag2Se Turing pattern on the CoSe2 surface.
Further research found that this multi-interface Turing structure Ag2Se-CoSe2 material is an efficient oxygen evolution electrocatalyst.
It only needs an overvoltage of 221mV to achieve an oxygen generation current density of 10mA/cm2, and its anode oxygen evolution efficiency is 84.
5%.
Experiments show that the OER activity of Ag2Se-CoSe2 is linearly related to the interface length of the Turing structure.
The rich interface structure and the optimized adsorption energy of the intermediate at the interface structure are the main reasons for its high activity.
This research uses the "reaction-diffusion" theory to build a complex Turing structure on inorganic nanostructured materials for the first time, providing new ideas for the design of cheaper catalysts with higher performance.