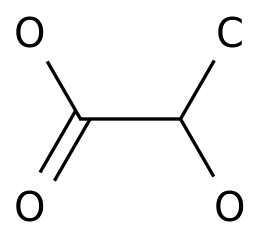
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On June 19, according to US media reports, from electric vehicles that can travel hundreds of miles on a single charge, to chain saws as powerful as gasoline saws, new products that take advantage of the latest advances in battery technology enter the market
every year.
But this growth momentum has led to fears that the world's lithium supply may eventually run out
.
Lithium is a metal that is the core material
of many new rechargeable batteries.
Now, researchers at the Georgia Institute of Technology have found new evidence
that sodium- and potassium-based batteries promise to be a potential alternative to lithium batteries.
Matthew McDowell, assistant professor at the George W.
Woodruff School of Mechanical Engineering and the School of Materials Science and Engineering, said: "One of the biggest hurdles for sodium- and potassium-ion batteries is that they tend to decay and age faster and store less
energy than other batteries.
But we found that this wasn't always the case
.
The research team studied how three different ions, lithium, sodium and potassium, react with iron sulfide particles
.
The study, which was funded by the National Science Foundation and the Department of Energy, was published June 19 in
the journal Joule.
As the battery is charged and discharged, ions constantly react with the particles that make up the battery electrodes and penetrate through them
.
This reaction process causes a large number of changes in the electrode particles, often crushing them into fine particles
.
Because sodium and potassium ions are larger than lithium ions, they have traditionally been thought to cause more severe aging
when they react with particles.
In the experiment, they directly observed the reaction occurring inside the battery under an electron microscope, in which iron sulfide particles act as
battery electrodes.
The researchers found that iron sulfide that reacted with sodium and potassium ions was more stable than iron sulfide that reacted with lithium ions, suggesting that sodium- or potassium-based battery life may be much
longer than expected.
On June 19, according to US media reports, from electric vehicles that can travel hundreds of miles on a single charge, to chain saws as powerful as gasoline saws, new products that take advantage of the latest advances in battery technology enter the market
every year.
But this growth momentum has led to fears that the world's lithium supply may eventually run out
.
Lithium is a metal that is the core material
of many new rechargeable batteries.
Now, researchers at the Georgia Institute of Technology have found new evidence
that sodium- and potassium-based batteries promise to be a potential alternative to lithium batteries.
Matthew McDowell, assistant professor at the George W.
Woodruff School of Mechanical Engineering and the School of Materials Science and Engineering, said: "One of the biggest hurdles for sodium- and potassium-ion batteries is that they tend to decay and age faster and store less
energy than other batteries.
But we found that this wasn't always the case
.
The research team studied how three different ions, lithium, sodium and potassium, react with iron sulfide particles
.
The study, which was funded by the National Science Foundation and the Department of Energy, was published June 19 in
the journal Joule.
As the battery is charged and discharged, ions constantly react with the particles that make up the battery electrodes and penetrate through them
.
This reaction process causes a large number of changes in the electrode particles, often crushing them into fine particles
.
Because sodium and potassium ions are larger than lithium ions, they have traditionally been thought to cause more severe aging
when they react with particles.
In the experiment, they directly observed the reaction occurring inside the battery under an electron microscope, in which iron sulfide particles act as
battery electrodes.
The researchers found that iron sulfide that reacted with sodium and potassium ions was more stable than iron sulfide that reacted with lithium ions, suggesting that sodium- or potassium-based battery life may be much
longer than expected.