-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
-
Cosmetic Ingredient
- Water Treatment Chemical
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
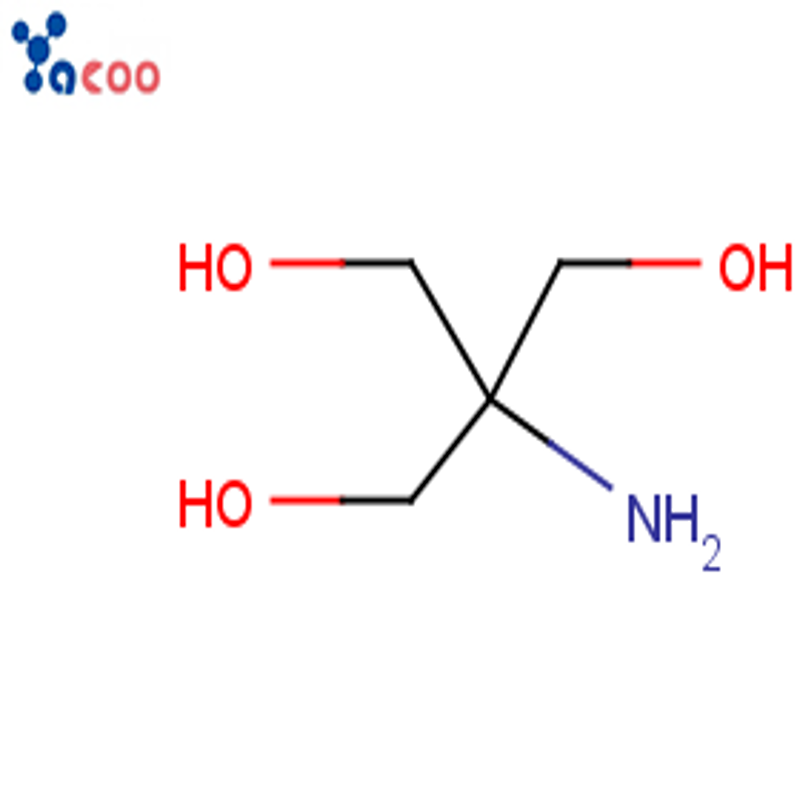
Bis(4-amino-3-methylcyclohexyl)methane, also known as BMAH, is an important intermediate chemical used in the production of a variety of products in the chemical industry.
It is used in the production of polyurethanes, which are widely used in the manufacture of furniture, automobiles, and other consumer products.
BMAH is also used in the production of pigments, dyes, and other colorants.
There are several synthetic routes to produce BMAH, which can be broadly classified into two categories: direct and indirect routes.
- Direct Route: The direct route involves the reaction of 4-amino-3-methylcyclohexanone with methyl iodide in the presence of a base such as sodium hydroxide.
The reaction results in the formation of BMAH, with the elimination of water and iodide.
This method is straightforward and relatively simple, but it requires the handling of hazardous reagents such as iodine and methyl iodide. - Indirect Route: The indirect route involves the synthesis of 4-amino-3-methylcyclohexanone, which is then converted to BMAH using a series of chemical reactions.
The synthesis of 4-amino-3-methylcyclohexanone can be achieved through several methods, including the hydrolysis of 4-chloro-3-methylcyclohexane, the reduction of 4-bromo-3-methylcyclohexanone, or the reaction of 4-nitro-3-methylcyclohexanone with ammonia.
The indirect route is generally considered to be safer and more cost-effective than the direct route, as it eliminates the need for hazardous reagents and simplifies the production process.
However, the indirect route also requires additional steps and chemical reagents, which can increase the overall complexity of the process.
One of the most commonly used indirect routes to BMAH involves the synthesis of 4-amino-3-methylcyclohexanone from 4-chloro-3-methylcyclohexane, which is then converted to BMAH using a series of chemical reactions.
The synthesis of 4-amino-3-methylcyclohexanone from 4-chloro-3-methylcyclohexane involves the hydrolysis of the chloride with a strong base such as sodium hydroxide.
This results in the formation of 4-amino-3-methylcyclohexanone, which can then be purified and used in the subsequent steps of the process.
BMAH can be produced from 4-amino-3-methylcyclohexanone using a variety of chemical reactions and reagents.
One common method involves the reaction of 4-amino-3-methylcyclohexanone with a strong acid such as hydrochloric acid in the presence of a solvent such as dichloromethane.
This reaction results in the formation of the bis(4-amino-3-methylcyclohexyl)methane, with the elimination of water and the formation of a purchased sodium chloride.
Another method for the production of BMAH involves the reaction of 4-amino-3-methylcyclohexanone with methyl iodide in the presence of a base such as sodium hydroxide.
This reaction results in the formation of BMAH, with the elimination of water and iodide.
In conclusion, Bis(4-amino-3-methylcyclohexyl)methane is an important intermediate chemical used in the production of a variety of products in the chemical industry.
There are several synthetic routes to produce BMAH, which can be broadly classified into two