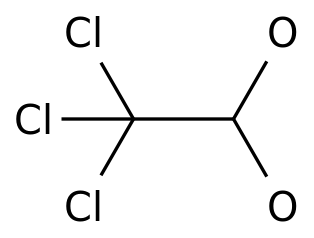
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read and refer to Professor Zhou Dong's personal explanation, which is full of rewards
.
At the 24th National Neurology Conference of the Chinese Medical Association held on September 24-26, 2021, Professor Zhou Dong from West China Hospital of Sichuan University shared with us the progress of epilepsy drugs in three aspects.
, This article has made a summary for everyone, hurry up and learn! 1.
New antiepileptic drugs and evidence.
During the eight years of the Chinese New Year, a total of 8 new drugs have been marketed worldwide, but only 3 new drugs have been marketed in China, namely Lacosamide, Perampanel, and Perampanel.
Pregabalin (Pregabalin)
.
In addition, since 2015, there have been 5 new drugs on the market around the world, which are widely used abroad, but China is still 0 on the market.
Next, Professor Zhou Dong introduced these 5 new drugs for us
.
1.
Brivaracetam: 11 years of open follow-up study results 11 years of open label follow-up of 667 patients with epilepsy (focal origin); the highest 200mg/d Brivaracetam addition therapy is well tolerated; 55.
6% Patients reported a 50% reduction in seizures, and 30.
3% reported a reduction in seizures over 6 months; there was an improvement in the efficacy of the application time, and the side effects of the drug were also significantly lower than that of levetiracetam
.
2.
Cannabidiol (Cannabidiol) 1.
The dose range study of cannabidiol in Dravet syndrome.
Randomized double-blind clinical controlled trial: Included in 6 countries, 38 centers, 199 patients with confirmed Dravet [baseline seizure frequency more than 4 times comprehensive Tonic-clonic seizures (GTCS)/4 weeks, monotherapy failure]; 10mg and 20mg/kg/d group cannabidiol oral add-on treatment, similar to the reduction of seizure frequency, the 10mg group is better than the 20mg group in safety and tolerability ; The addition of cannabidiol exceeds 10mg/kg/d, and individualized effectiveness and safety should be weighed
.
2.
The effect of cannabidiol on the treatment of epilepsy was included in 4 randomized controlled trials (RCT) double-blind (550 patients) and 3 prospective open trials (more than 1,000 patients); in the "severe" cases of tonic clonus, rigidity, etc.
In the type of seizures, it is beneficial to reduce seizures; cannabidiol may be effective in drug-resistant epilepsy other than Dravet and LG syndrome
.
3.
Carbamate (Cenobamate): Multi-center, double-blind, controlled RCT recruited 437 epilepsy patients from 107 centers in 16 countries and randomly divided into four groups of placebo/100mg/200mg/400mg and followed up for 18 weeks; compared with the placebo group In comparison, the median percentage of seizure frequency reduction in each experiment was 24%, 35.
5% in the 100 mg group, and 55.
0% in the 200/400 mg group; the clinical retention rate was significantly related to the increase in drug dose, and the 200 mg/400 mg group had significant epilepsy during the maintenance period.
Onset efficiency
.
4.
Everolimus (Everolimus): retrospective study of adult tuberous sclerosis (TSC) retrospective study of adult tuberous sclerosis (TSC) patients, the use of Everolimus (Everolimus, EVE) for more than 18 years old; the results show that EVE It is an effective add-on treatment for patients with epilepsy and TSC, and there is no age limit for the benefit; in 45 patients, side effects are more common, including 3 deaths [2 suspicious epilepsy sudden death (SUDEP), 1 renal hemangioma acute hemorrhage)]; Close attention should be paid to the side effects of adult patients: infections and oral inflammations are common
.
Fenfluramine (Fenfluramine): Two RCTs of new drugs for Dravet syndrome confirmed that Fenfluramine can effectively treat Dravet syndrome, and the reduction rate of direct convulsions in the placebo group is as high as 54%-62%
.
Fenfluramine has been used to lose weight in obese patients, and it has cardiotoxicity; neither of the above two RCTs reported cardiotoxicity
.
[Possible new targets for drug therapy] 1.
Deep brain stimulation, optogenetics, chemical genetics and other precise and dare methods will further reveal the core target of the epileptic brain network: deep brain stimulation (DBS) photochemical DREADDS (chemical Genetic) Minimally Invasive Electrical Stimulation 2.
Possibility of Loop Drugs: Electroreactive Hydrogel Nanoparticles Precision Restricted Drugs (DART) 4*Spefic Antiepileptic Drugs 2.
New Evidence for Drug Application 1.
2 The SANADII study published on LANCET in 2002 :Evaluation of long-term clinical effectiveness and cost of initial medication for patients with epilepsy: Levetiracetam (LEV) vs.
Valproate (VPA); LEV compared with VPA.
The 12-month seizure remission did not meet the definition of non-inferiority, and the treatment failure time, the 2-year seizure remission rate, and the first and subsequent seizures were worse; the results suggest that LEV as the first-line treatment for patients with epilepsy of full origin or unclassified has no clinical effect And cost advantage
.
However, the impact of VPA should still be discussed for women of childbearing age
.
2.
Carbamazepine (CBZ) and oxcarbazepine (OXC) are used in focal origin epilepsy with the continuous emergence of new anti-epileptic drugs, changing the drug distribution in the guidelines, but currently CBA and OXC are still major drug guidelines in Europe, America and China First-line and second-line treatment options for epilepsy of focal origin and general tonic-clonic seizures; In resource-poor areas, CBZ is still one of the most commonly prescribed prescriptions for epilepsy of focal origin, and attention should be paid to drug-related effects and side effects; In the United States, China and other regions, OXC has gradually replaced CBZ as the first-line drug regimen for focal-origin seizures, and it is better tolerated; the results do not support LEV and ZNS as the first-line treatment for patients with focal-origin epilepsy, and LTG is still the comparison standard for future trials
.
3.
Single-center retrospective cohort study (2006-2016) of anti-epileptic drug therapy (single drug failure and drug dose ≥50% DDD) after the drug dose reached the standard; the seizure-free rate of the combined treatment group was significantly higher than that of single drug replacement therapy Group and increased-dose group; combination therapy uses more broad-spectrum antiepileptic drugs
.
4.
Drug treatment strategies for drug-resistant epilepsy (disease> 2 years and failure of two or more anti-epileptic drugs) single-center prospective cohort study (2010-2018); add or switch to existing anti-epileptic drugs New types of drugs, but the addition or switching to epilepsy drugs did not differ in seizure-related outcomes; there was no difference in discontinuation due to side effects between the two groups
.
5.
In the 30 years since newly diagnosed patients with epilepsy are resistant to antiseizure drugs, the use of second-generation antiseizure drugs has increased, and the overall treatment tolerance of newly diagnosed patients has not increased significantly; nervous system abnormalities and nervous system abnormalities have increased, and the incidence of skin rash has increased from 8.
11 % Decreased to 4.
15%; the number of seizures before treatment, the types of drugs that were previously discontinued due to intolerable adverse reactions (AEs), the number of combination drugs, and female patients are risk factors for poor tolerance of antiepileptic drugs
.
6.
Data from phase 2 and phase 3 randomized clinical controlled trials of drugs related to suicide risk caused by new anti-epileptic drugs, including 4000 patients in the anti-epileptic drug group and 1996 controls; currently there is no evidence that these new anti-epileptic drugs increase the risk of suicide; Is it necessary to continue to maintain suicide risk warnings for these drugs? 7.
Verification and comparison of drug withdrawal and recurrence prediction models in the Wenzhou epilepsy follow-up database for 212 withdrawn patients to verify the two models; in the external verification, the Lamberink 2-year model has the best AUC (0.
71); the Lamberink 2-year model (P= 0.
121) and the MRC 1-year model (P=0.
264) are well calibrated; in the decision curve analysis, the Lamberink 2-year model performs well when the threshold probability is 30%-65%
.
3.
Analysis of real world drug treatment in China 1.
National multi-center real world study 1) Scope of inclusion: 127 tertiary medical units in 31 provinces and cities across the country 2) Time of inclusion: July 1, 2017 to December 31, 2020 3 ) A total of 15,535 hospitalized patients with epilepsy diagnosed on the online platform were included, with an average age of 34.
50 years old and a median age of 30 years old.
The proportion of men was 59.
11%, and 86.
92% of the patients were Han nationality
.
4) Regular use rate of anti-epileptic drugs: 72.
01% of patients who have been diagnosed with more than 3 months of regular use of anti-epileptic drugs in the past 3 months; the number of people who have used various commonly used anti-epileptic drugs and nearly 3 The ratio of monthly regular medications, that is, the retention rate, is less than 75%.
5) The rate of serious adverse events of anti-epileptic drugs has continued to decline in the past 3 years, which has dropped from 4.
44% to 1.
28% 2.
Sodium valproate Comparison of the efficacy of drug treatment after drug treatment failed.
Retrospectively included 2656 patients with epilepsy after the failure of the first drug of VPA who were followed up in West China Hospital of Sichuan University from 2009 to 2019.
According to the type of seizures, they were divided into focal origin (N=1144), comprehensive Three groups of origin (N=1093) and unknown origin (N=419)
.
The types of added drugs include: levetiracetam, lamotrigine, oxcarbazepine, topiramate, carbamazepine
.
In the focal origin group, the total effective rate of the VPA+OXC group was higher than that of the other groups, but there was no significant difference compared with the VPA+LTG group; the total effective rate of the comprehensive origin group, the VPA+LTG group was higher than the other four groups and was statistically significant Significance: In the unknown origin group, the total effective rate of the VPA+LEV group is higher than the other four groups, but the comparison between the groups is not statistically significant
.
3.
The change in the number of medications used by Chinese women with epilepsy during pregnancy comes from the use of medications and seizures in 1742 patients from West China Pregnancy Registry; the proportion of those who did not use medications during pregnancy in China has decreased year by year, and is now equivalent to the Australian Pregnancy Registry (9.
8% vs.
7.
9%); The proportion of monotherapy is increasing year by year, and in 2020, the proportion of monotherapy is 52.
3%
.
4.
Changes in the types of single-drug treatments for women with epilepsy in China.
The proportion of using CBZ, VPA, and TPM during pregnancy is decreasing year by year.
Among them, VPA and CBZ have decreased the most.
In 2020, the proportions of single-drug treatment of VPA and CBZ in pregnancy are 7.
1% and 8.
5%, respectively.
; The proportion of using LEV, LTG, and OXC is increasing year by year.
Among them, the increasing trend of LEV is the most obvious.
In 2020, the proportion of LEV monotherapy is 46.
8%
.
5.
The control of epilepsy in Chinese women with epilepsy during pregnancy.
The proportion of women with seizure seizures during pregnancy has gradually decreased (52.
07% in 2013 vs.
30.
30% in 2020); the proportion of authors without epilepsy has increased year by year (24.
29% vs.
2013) (43.
36% in 2020); the proportion of non-convulsive seizures during pregnancy is relatively stable, fluctuating around 25%
.
Summary: Finally, Professor Zhou Dong pointed out that although the current evidence of new anti-epileptic drugs and medications continues to emerge; anti-epileptic seizures and drug resistance targets are gradually becoming clear, it is still necessary to further promote the standardization process of drugs in China and make a Chinese voice! The content of this article is compiled from the lecture of Professor Zhou Dong at the 24th National Neurology Conference of the Chinese Medical Association-"Progress in Drug Therapy for Epilepsy", the picture is from the professor's PPT
.
.
At the 24th National Neurology Conference of the Chinese Medical Association held on September 24-26, 2021, Professor Zhou Dong from West China Hospital of Sichuan University shared with us the progress of epilepsy drugs in three aspects.
, This article has made a summary for everyone, hurry up and learn! 1.
New antiepileptic drugs and evidence.
During the eight years of the Chinese New Year, a total of 8 new drugs have been marketed worldwide, but only 3 new drugs have been marketed in China, namely Lacosamide, Perampanel, and Perampanel.
Pregabalin (Pregabalin)
.
In addition, since 2015, there have been 5 new drugs on the market around the world, which are widely used abroad, but China is still 0 on the market.
Next, Professor Zhou Dong introduced these 5 new drugs for us
.
1.
Brivaracetam: 11 years of open follow-up study results 11 years of open label follow-up of 667 patients with epilepsy (focal origin); the highest 200mg/d Brivaracetam addition therapy is well tolerated; 55.
6% Patients reported a 50% reduction in seizures, and 30.
3% reported a reduction in seizures over 6 months; there was an improvement in the efficacy of the application time, and the side effects of the drug were also significantly lower than that of levetiracetam
.
2.
Cannabidiol (Cannabidiol) 1.
The dose range study of cannabidiol in Dravet syndrome.
Randomized double-blind clinical controlled trial: Included in 6 countries, 38 centers, 199 patients with confirmed Dravet [baseline seizure frequency more than 4 times comprehensive Tonic-clonic seizures (GTCS)/4 weeks, monotherapy failure]; 10mg and 20mg/kg/d group cannabidiol oral add-on treatment, similar to the reduction of seizure frequency, the 10mg group is better than the 20mg group in safety and tolerability ; The addition of cannabidiol exceeds 10mg/kg/d, and individualized effectiveness and safety should be weighed
.
2.
The effect of cannabidiol on the treatment of epilepsy was included in 4 randomized controlled trials (RCT) double-blind (550 patients) and 3 prospective open trials (more than 1,000 patients); in the "severe" cases of tonic clonus, rigidity, etc.
In the type of seizures, it is beneficial to reduce seizures; cannabidiol may be effective in drug-resistant epilepsy other than Dravet and LG syndrome
.
3.
Carbamate (Cenobamate): Multi-center, double-blind, controlled RCT recruited 437 epilepsy patients from 107 centers in 16 countries and randomly divided into four groups of placebo/100mg/200mg/400mg and followed up for 18 weeks; compared with the placebo group In comparison, the median percentage of seizure frequency reduction in each experiment was 24%, 35.
5% in the 100 mg group, and 55.
0% in the 200/400 mg group; the clinical retention rate was significantly related to the increase in drug dose, and the 200 mg/400 mg group had significant epilepsy during the maintenance period.
Onset efficiency
.
4.
Everolimus (Everolimus): retrospective study of adult tuberous sclerosis (TSC) retrospective study of adult tuberous sclerosis (TSC) patients, the use of Everolimus (Everolimus, EVE) for more than 18 years old; the results show that EVE It is an effective add-on treatment for patients with epilepsy and TSC, and there is no age limit for the benefit; in 45 patients, side effects are more common, including 3 deaths [2 suspicious epilepsy sudden death (SUDEP), 1 renal hemangioma acute hemorrhage)]; Close attention should be paid to the side effects of adult patients: infections and oral inflammations are common
.
Fenfluramine (Fenfluramine): Two RCTs of new drugs for Dravet syndrome confirmed that Fenfluramine can effectively treat Dravet syndrome, and the reduction rate of direct convulsions in the placebo group is as high as 54%-62%
.
Fenfluramine has been used to lose weight in obese patients, and it has cardiotoxicity; neither of the above two RCTs reported cardiotoxicity
.
[Possible new targets for drug therapy] 1.
Deep brain stimulation, optogenetics, chemical genetics and other precise and dare methods will further reveal the core target of the epileptic brain network: deep brain stimulation (DBS) photochemical DREADDS (chemical Genetic) Minimally Invasive Electrical Stimulation 2.
Possibility of Loop Drugs: Electroreactive Hydrogel Nanoparticles Precision Restricted Drugs (DART) 4*Spefic Antiepileptic Drugs 2.
New Evidence for Drug Application 1.
2 The SANADII study published on LANCET in 2002 :Evaluation of long-term clinical effectiveness and cost of initial medication for patients with epilepsy: Levetiracetam (LEV) vs.
Valproate (VPA); LEV compared with VPA.
The 12-month seizure remission did not meet the definition of non-inferiority, and the treatment failure time, the 2-year seizure remission rate, and the first and subsequent seizures were worse; the results suggest that LEV as the first-line treatment for patients with epilepsy of full origin or unclassified has no clinical effect And cost advantage
.
However, the impact of VPA should still be discussed for women of childbearing age
.
2.
Carbamazepine (CBZ) and oxcarbazepine (OXC) are used in focal origin epilepsy with the continuous emergence of new anti-epileptic drugs, changing the drug distribution in the guidelines, but currently CBA and OXC are still major drug guidelines in Europe, America and China First-line and second-line treatment options for epilepsy of focal origin and general tonic-clonic seizures; In resource-poor areas, CBZ is still one of the most commonly prescribed prescriptions for epilepsy of focal origin, and attention should be paid to drug-related effects and side effects; In the United States, China and other regions, OXC has gradually replaced CBZ as the first-line drug regimen for focal-origin seizures, and it is better tolerated; the results do not support LEV and ZNS as the first-line treatment for patients with focal-origin epilepsy, and LTG is still the comparison standard for future trials
.
3.
Single-center retrospective cohort study (2006-2016) of anti-epileptic drug therapy (single drug failure and drug dose ≥50% DDD) after the drug dose reached the standard; the seizure-free rate of the combined treatment group was significantly higher than that of single drug replacement therapy Group and increased-dose group; combination therapy uses more broad-spectrum antiepileptic drugs
.
4.
Drug treatment strategies for drug-resistant epilepsy (disease> 2 years and failure of two or more anti-epileptic drugs) single-center prospective cohort study (2010-2018); add or switch to existing anti-epileptic drugs New types of drugs, but the addition or switching to epilepsy drugs did not differ in seizure-related outcomes; there was no difference in discontinuation due to side effects between the two groups
.
5.
In the 30 years since newly diagnosed patients with epilepsy are resistant to antiseizure drugs, the use of second-generation antiseizure drugs has increased, and the overall treatment tolerance of newly diagnosed patients has not increased significantly; nervous system abnormalities and nervous system abnormalities have increased, and the incidence of skin rash has increased from 8.
11 % Decreased to 4.
15%; the number of seizures before treatment, the types of drugs that were previously discontinued due to intolerable adverse reactions (AEs), the number of combination drugs, and female patients are risk factors for poor tolerance of antiepileptic drugs
.
6.
Data from phase 2 and phase 3 randomized clinical controlled trials of drugs related to suicide risk caused by new anti-epileptic drugs, including 4000 patients in the anti-epileptic drug group and 1996 controls; currently there is no evidence that these new anti-epileptic drugs increase the risk of suicide; Is it necessary to continue to maintain suicide risk warnings for these drugs? 7.
Verification and comparison of drug withdrawal and recurrence prediction models in the Wenzhou epilepsy follow-up database for 212 withdrawn patients to verify the two models; in the external verification, the Lamberink 2-year model has the best AUC (0.
71); the Lamberink 2-year model (P= 0.
121) and the MRC 1-year model (P=0.
264) are well calibrated; in the decision curve analysis, the Lamberink 2-year model performs well when the threshold probability is 30%-65%
.
3.
Analysis of real world drug treatment in China 1.
National multi-center real world study 1) Scope of inclusion: 127 tertiary medical units in 31 provinces and cities across the country 2) Time of inclusion: July 1, 2017 to December 31, 2020 3 ) A total of 15,535 hospitalized patients with epilepsy diagnosed on the online platform were included, with an average age of 34.
50 years old and a median age of 30 years old.
The proportion of men was 59.
11%, and 86.
92% of the patients were Han nationality
.
4) Regular use rate of anti-epileptic drugs: 72.
01% of patients who have been diagnosed with more than 3 months of regular use of anti-epileptic drugs in the past 3 months; the number of people who have used various commonly used anti-epileptic drugs and nearly 3 The ratio of monthly regular medications, that is, the retention rate, is less than 75%.
5) The rate of serious adverse events of anti-epileptic drugs has continued to decline in the past 3 years, which has dropped from 4.
44% to 1.
28% 2.
Sodium valproate Comparison of the efficacy of drug treatment after drug treatment failed.
Retrospectively included 2656 patients with epilepsy after the failure of the first drug of VPA who were followed up in West China Hospital of Sichuan University from 2009 to 2019.
According to the type of seizures, they were divided into focal origin (N=1144), comprehensive Three groups of origin (N=1093) and unknown origin (N=419)
.
The types of added drugs include: levetiracetam, lamotrigine, oxcarbazepine, topiramate, carbamazepine
.
In the focal origin group, the total effective rate of the VPA+OXC group was higher than that of the other groups, but there was no significant difference compared with the VPA+LTG group; the total effective rate of the comprehensive origin group, the VPA+LTG group was higher than the other four groups and was statistically significant Significance: In the unknown origin group, the total effective rate of the VPA+LEV group is higher than the other four groups, but the comparison between the groups is not statistically significant
.
3.
The change in the number of medications used by Chinese women with epilepsy during pregnancy comes from the use of medications and seizures in 1742 patients from West China Pregnancy Registry; the proportion of those who did not use medications during pregnancy in China has decreased year by year, and is now equivalent to the Australian Pregnancy Registry (9.
8% vs.
7.
9%); The proportion of monotherapy is increasing year by year, and in 2020, the proportion of monotherapy is 52.
3%
.
4.
Changes in the types of single-drug treatments for women with epilepsy in China.
The proportion of using CBZ, VPA, and TPM during pregnancy is decreasing year by year.
Among them, VPA and CBZ have decreased the most.
In 2020, the proportions of single-drug treatment of VPA and CBZ in pregnancy are 7.
1% and 8.
5%, respectively.
; The proportion of using LEV, LTG, and OXC is increasing year by year.
Among them, the increasing trend of LEV is the most obvious.
In 2020, the proportion of LEV monotherapy is 46.
8%
.
5.
The control of epilepsy in Chinese women with epilepsy during pregnancy.
The proportion of women with seizure seizures during pregnancy has gradually decreased (52.
07% in 2013 vs.
30.
30% in 2020); the proportion of authors without epilepsy has increased year by year (24.
29% vs.
2013) (43.
36% in 2020); the proportion of non-convulsive seizures during pregnancy is relatively stable, fluctuating around 25%
.
Summary: Finally, Professor Zhou Dong pointed out that although the current evidence of new anti-epileptic drugs and medications continues to emerge; anti-epileptic seizures and drug resistance targets are gradually becoming clear, it is still necessary to further promote the standardization process of drugs in China and make a Chinese voice! The content of this article is compiled from the lecture of Professor Zhou Dong at the 24th National Neurology Conference of the Chinese Medical Association-"Progress in Drug Therapy for Epilepsy", the picture is from the professor's PPT
.