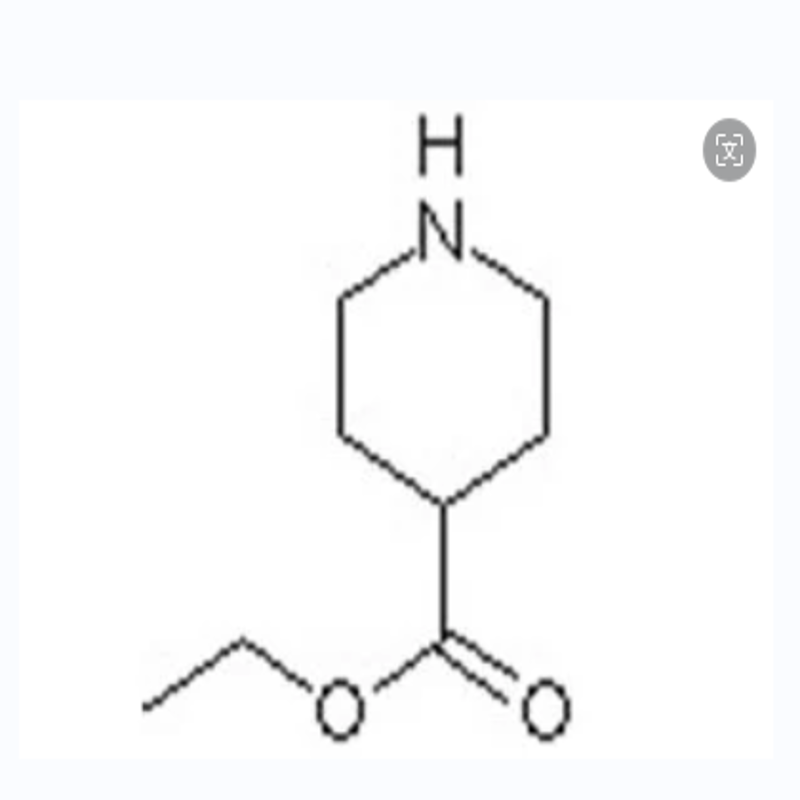
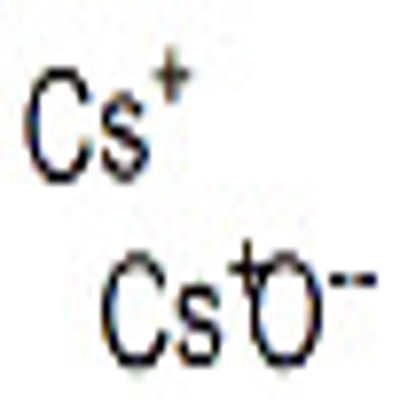-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On the 13th, Apellis Pharmaceuticals announced the detailed results of the C3 complementary targeted therapy pegcetacoplan, developed by the company at the 25th annual meeting of the European Society of Hematology, in a critical Phase 3 clinical trial for the treatment of patients with pontox sleep hemoglobinin (PNH)The results showed that pegcetacoplan reached the main end point of the trial and significantly increased the patient's hemoglobin level compared to the approved therapyMoreover, pegcetacoplan also showed superiority in several other indicators that measured a patient's responseThe company plans to submit listing applications to Regulators in the United States and the European Union later this yearPNH is a life-threatening rare blood disease characterized by the destruction of red blood cells (hemolytic anemia), blood clots (thrombosis), impaired bone marrow function, and the risk of leukemiaThe disease is due to a genetic mutation that causes the key CD55 and CD59 receptors that interact with the alternative pathway (AP) that interacts with the complement cascade reaction, causing the complementary reaction seisphon seismost stimulated by the alternative pathway to destroy the red blood cells through an intravascular hemolytic actionCurrently, the main treatment for PNH is to inhibit the C5 protein through C5 supplement inhibitorsHowever, in the course of treatment with C5 inhibitors, up to 75% of PNH patients develop anemia, and some patients also need to receive blood transfusion treatmentApellis developed pegcetacoplan as a C3 supplement inhibitor designed to regulate excessive recombination activation in many diseasesPegcetacoplan is a synthetic ring-like peptide that is coupled with polyglycol polymers and can bind specifically to C3 and C3bPreviously, the FDA has granted pegcetacoplan fast-track eligibility for PNH and GA patientsAccording to Apellis, pegcetacoplan has the potential to address the limitations of existing therapies as treatment options for "best-in-class" and "first-in-class"'s Role Mechanism (Photo: Apellis)trial called PEGASUS is a phase 3 clinical study of 80 adult PNH patients with active control groupsThe patient was first treated with a combination of 4 weeks of pegcetacoplan and eculizumab, and then randomly treated with eculizumab or pegcetacoplanThe results showed that after 16 weeks of treatment, the average hemoglobin level in patients treated with pegcetacoplan improved by 3.8 g/dL compared to those in the active control group, reaching the primary endpoint of the trialin addition, 85% of patients in the pegcetacoplan treatment group did not need blood transfusion treatment during the 16-week course, compared with 15% in the active control group The proportion of patients with normal levels of lactic acid dehydrogenase (LDH) in the Pegcetacoplan treatment group reached 71% and the active control group 15% Moreover, using the FACIT fatigue score, the patients in the pegcetacoplan group scored 11 points better than the control group "Even with current treatment, anemia and endless fatigue caused by the extravascular hemolytic blood driven by the C3 supplement remain a serious problem for PNH patients." Dr Peter Hillmen, professor of experimental hematology at the University of Leeds, said: 'These results demonstrate that pegcetacoplan controls blood outside and intravascular hemolytic blood, with the potential to become the new standard treatment for PNH "
References: Retrieved D I6 12, 2020, from Superior Data Demonstrateity of Apellis' Pegcetacoplan to Eculizumab in Improving Hemoglobin Levels, Independent Prior of The Dre Thor, in PNH.