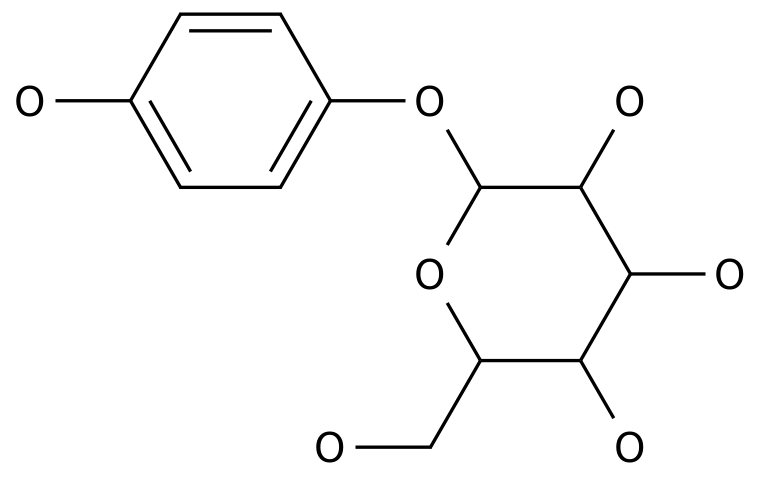
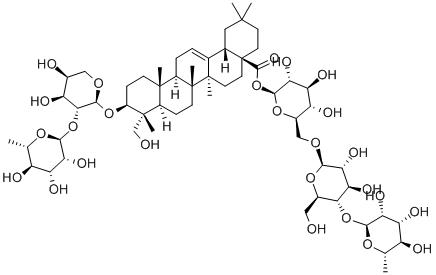
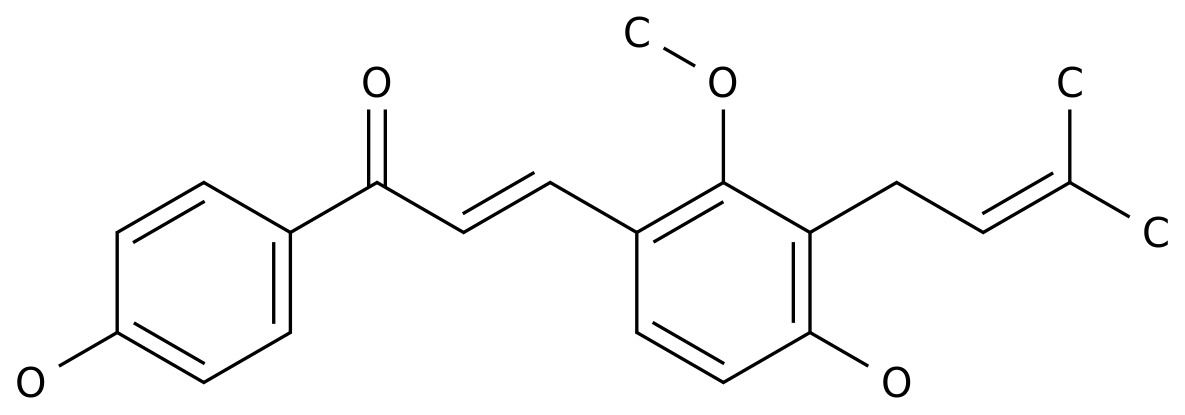
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The technology for plant-made pharmaceuticals (PMPs) has progressed significantly over the last few years, with the first commercial products for human use expected to reach the market by 2009 (
see
Note
1
). As part of the ‘next generation’ of genetically modified (GM) crops, PMPs will be subject to additional biosafety considerations and are set to challenge the complex and overlapping regulations that currently govern GM plants, plant biologics (
see
Note
2
) and ‘conventional’ pharmaceutical production. The areas of responsibility are being mapped out between the different regulatory agencies (Sparrow, P.A.C., Irwin, J., Dale, P., Twyman, R.M., and Ma, J.K.C. (2007) Pharma-Planta: Road testing the developing regulatory guidelines for plant-made pharmaceuticals.
Transgenic Res
., 2007), with specific guidelines currently being drawn up for the regulation of PMPs. In this chapter, we provide an overview of the biosafety (
see
Note
3
), risk assessment (
see
Note
4
) and regulation of this emerging technology. While reference will be made to EU regulations, the underlying principles of biosafety and risk assessment are generic to most countries.