-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read and refer to the exciting content of the three major academic specials of CNF 2021 Bojian
.
On October 29-31, 2021, the 15th China Neurology Forum (CNF 2021) meeting was held in Shanghai
.
Among them, Bojian Biological, as a pioneer in the field of neuroscience, hosted the academic special sessions of the three satellite conferences of multiple sclerosis, spinal muscular atrophy, and Alzheimer's disease to share the latest research progress and clinical diagnosis and treatment experience in the field of neurological diseases
.
The three academic sessions invited Professor Dong Qiang from Huashan Hospital Affiliated to Fudan University and Professor Luo Benyan from the First Affiliated Hospital of Zhejiang University School of Medicine as chairpersons.
Professor Liu Xueyuan from the Tenth People's Hospital of the city gave wonderful reports on related topics
.
Next, the editor will take everyone to briefly review the essence of the academic feast
.
2 major new MS drugs to help patients regain control of their out-of-control lives.
Professor Chen Xiangjun, Huashan Hospital, Fudan University.
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system.
Most people may be relatively uncomfortable with this rare disease.
Relatively unfamiliar, but in fact, in recent years, the global prevalence of MS has shown an increasing trend.
The prevalence in mainland China has increased by nearly 50% between 1990 and 2016
.
As of 2016, the number of MS patients in Mainland China has exceeded 100,000
.
The life of MS patients is out of control.
As the disease progresses and relapses, the patient’s nervous system continues to be damaged.
They may gradually lose their ability to take care of themselves, become blind or even lose their lives.
.
.
The high disability of MS often prevents them from being normal.
According to the 2020 Comprehensive Social Survey of MS Patients in China, as many as 88.
5% of the respondents were unemployed or out of school due to MS; more than 43% of patients cannot take care of themselves completely and need care by others
.
Different from the embarrassing situation that patients with other rare diseases face "it is difficult to seek medical treatment, and it is even more difficult to seek medicine", MS is still a rare disease with medicines available, and the remission period is the key treatment period for MS.
Domestic and foreign guidelines/consensus recommendations Disease modification therapy (DMT) should be initiated as soon as possible during the remission period of MS
.
However, the latest data shows that the current DMT treatment rate in China is only 18%; and the existing DMT drugs in China mainly act on peripheral immunity, so drugs with new mechanisms of action are still needed to deal with MS heterogeneity
.
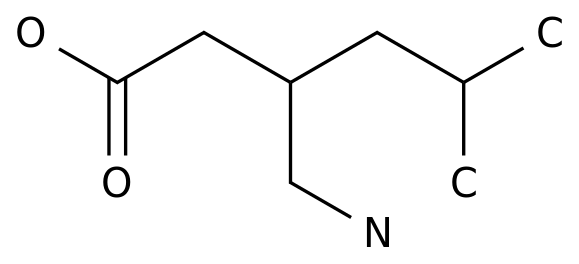
In April 2021, MS star new drug dimethyl fumarate was approved for marketing in China, bringing new treatment options for MS patients
.
■ Dimethyl fumarate: The world’s most prescribed MS oral DMT drug.
More than 500,000 patients receive treatment.
Dimethyl fumarate has a dual mechanism of action.
It can affect neurons, oligodendrocytes, Microglia and astrocytes have a direct effect to protect the central nervous system; at the same time, dimethyl fumarate regulates the peripheral immune system by regulating the number and function of different types of lymphocytes
.
In terms of efficacy, the comprehensive analysis of the Phase III study DEFINE and CONFIRM studies showed that the annual recurrence rate was reduced by 49% and the risk of disability progression was reduced by 32% after 2 years of dimethyl fumarate treatment
.
In addition, the ENDORSE study with a follow-up of up to 13 years showed that 45% of patients received dimethyl fumarate treatment for 10 years without recurrence, and 77% of patients were not restricted in walking
.
Figure 1: After receiving continuous dimethyl fumarate treatment for 10 years, 45% of patients have no recurrence, and 77% of patients have unrestricted walking.
In terms of safety, the common adverse reactions of dimethyl fumarate are mainly mild to moderate.
, Security is controllable
.
It is particularly worth mentioning that the initial medication of dimethyl fumarate does not require ECG monitoring and genetic testing, and its half-life is short, so there is no need to extend the washout period, and patients can flexibly arrange their birth plans
.
At present, many guidelines/consensus and authoritative journal reviews have listed dimethyl fumarate as the first-line DMT drug.
It is the world's most prescribed oral DMT drug for MS.
More than 500,000 patients have been treated with dimethyl fumarate.
Fully witnessed its efficacy and safety
.
Figure 2: Many guidelines/consensus and authoritative journal reviews have listed dimethyl fumarate as a first-line DMT drug.
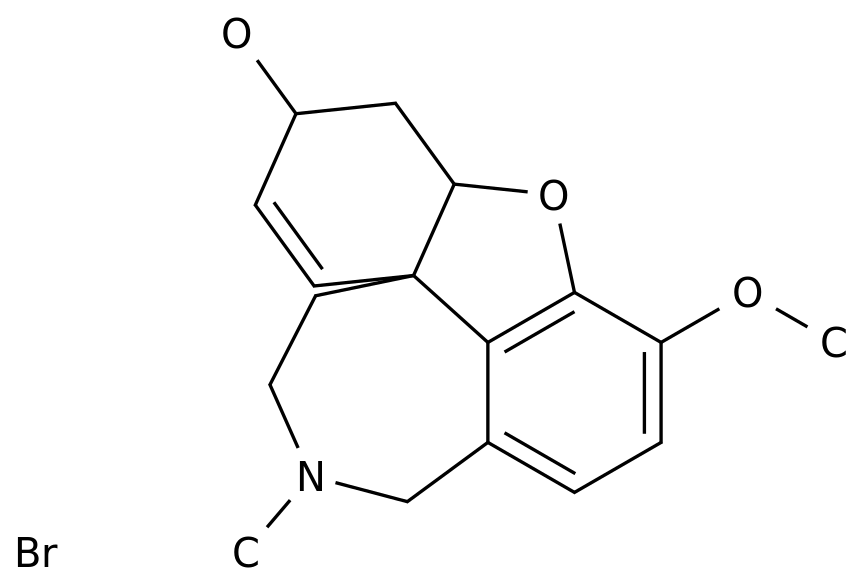
One month after dimethyl fumarate was approved in China, MS patients once again ushered in good news, Bojian Another blockbuster new biological drug, Fampridine Sustained-Release Tablets, was also successfully approved.
This time Bojian is targeting MS patients who have difficulty walking
.
Walking ability disorder is one of the key undesirable features of MS.
It can affect patients' physical and mental life and other aspects, including mental health, daily activities, quality of life, and autonomy.
Up to 70% of MS patients with walking difficulties said that walking difficulties are MS The most disturbing aspect
.
However, there is still a lack of effective treatment methods for improving walking disorders suitable for various stages of MS in the clinic.
Taking hormone therapy as an example, although it can alleviate the disease activity in the short term, it has no effect on the progression of long-term disability; while DMT therapy can delay the disease.
Progress, but unable to quickly and effectively symptomatically improve walking disorders
.
The emergence of Fampridine Sustained-Release Tablets made up for this deficiency
.
■ Fampridine sustained-release tablets: the first and currently the only drug approved to improve walking dysfunction in MS patients.
Fampridine sustained-release tablets is a voltage-dependent potassium channel blocker, which can be used as a single agent or combined with DMT Medications, physical therapy and other symptomatic therapies are used for all subtypes of MS patients.
It is the first and currently the only drug approved for the improvement of MS-related walking disorders
.
As of March this year, more than 402,000 patients worldwide have been treated with fampridine sustained-release tablets
.
Fampridine has comprehensive and sufficient randomized controlled and post-marketing studies in many countries
.
The summary analysis of the F203/204 study showed that no matter what type of MS and treatment, Fampridine sustained-release tablets can significantly improve the T25FW (timed 25-foot walking) walking response rate of MS patients
.
Figure 3: Fampridine can significantly improve the patient's T25FW walking response rate regardless of the type of MS and treatment.
In addition, the ENHANCE study shows that starting from the second week, Fampridine sustained-release tablets can significantly improve the patient's 12-item MS walking Table (MSWS-12) score, 24 weeks was significantly better than the placebo group
.
While the efficacy is excellent, the safety of Fampridine Sustained-Release Tablets also performed well.
Most of the adverse events were mild to moderate in severity, and no new adverse events or trends were observed after long-term treatment
.
The successive approvals of dimethyl fumarate and fampridine sustained-release tablets provide MS patients with a comprehensive treatment plan for short-term improvement of walking ability and long-term reduction of disease disability.
It is believed that it will help more patients to regain control of their out-of-control lives.
.
In the era of disease correction treatment, Nosinason is a strong guardian of SMA patients, Professor Jiang Haishan from Southern Medical University Nanfang Hospital shared online that spinal muscular atrophy (SMA) is due to the degenerative disease of spinal motor neurons, leading to skeletal muscle atrophy and general muscle atrophy The hereditary neuromuscular disease was included in China’s "First List of Rare Diseases" in 2018
.
It is estimated that there are currently 850 new patients in China each year, and the total number of patients is about 25,000.
However, the number of registered SMA patients is only about 3,000
.
Based on the age of onset and the maximum motor function achieved, the International SMA Alliance (ISMAC) system classifies SMA from heavier to lighter into 5 subtypes: 0-4
.
Because SMA is the main genetic cause of infant death, the diagnosis and treatment of infant SMA has received more attention, and it is believed that adult SMA patients are only a small part of the disease, and the condition is also lighter
.
In fact, all types of SMA are progressive diseases, and adult SMA patients can be type 1, type 2, type 3, and type 4
.
Figure 4: The composition of adult SMA patients In China, adult SMA patients account for a large proportion of the SMA population, but the diagnosis and treatment of adult SMA patients is more difficult than that of children
.
Although SMA does not lead to faster death of adult patients, as time goes by, the patient's mobility will decline, which will have a great impact on the patient's quality of life.
Therefore, receiving treatment as soon as possible is essential to stabilize the condition and improve the patient's prognosis
.
Unfortunately, there has been no effective treatment for SMA for a long time, and disease management is limited to auxiliary treatment methods such as respiratory support and nutritional support
.
It wasn't until 2016 that the world's first DMT drug Nosinasyn for the cause of SMA came out, the deadlock of "no medicines available" for SMA patients was broken, and the treatment of SMA entered the DMT era
.
Nosinasan is an antisense oligonucleotide (ASO) designed to increase the level of SMN protein by regulating the splicing of the SMN2 gene
.
Nosinasan is administered intrathecally, without crossing the blood-brain barrier, and directly delivers the drug to the cerebrospinal fluid around the spinal cord to give treatment where the disease originated
.
Nosignal was approved to enter China in February 2019, bringing hope to countless families of patients
.
The evidence chain of Nosinathan is complete.
A number of key clinical studies and real-world studies have covered a wide range of SMA patients, including infants, children, and adults of different ages, and have shown consistent efficacy and safety
.
Next, let's take a look at the data from the adult real-world study: The study conducted by Walter et al.
is the first published study to evaluate the efficacy of Noxinazan in the treatment of adult SMA patients
.
The results showed that noxinazan treatment can improve and stabilize the motor function and respiratory function of adult SMA patients, and its overall tolerance is good without serious adverse events
.
Figure 5: Nosinathan treatment can improve and stabilize the motor function and respiratory function of adult SMA patients.
The study conducted by Hagenacker et al.
is the largest real-world study conducted by Nosinathan in adult SMA patients.
There are 173 adult patients
.
The results showed that the patient’s motor function score (HFMSE) continued to increase with the prolonged treatment time, and 96% of the treated patients achieved clinically significant motor function improvement or stability.
The research results have been published in the top journal in the field of neurology, Leaf knife neurology" on
.
Figure 6: 96% of SMA patients treated with Nosinathan have achieved clinically significant improvement or stabilization of motor function.
At present, a number of real-world data have suggested that Nosinathan can stabilize the condition and improve the function of adult SMA patients.
, Change the natural course of disease
.
A wide range of SMA patients worldwide are receiving nosinathan, more than 10,000 cases, of which 33% are adult patients
.
The first treatment plan for a clear pathological mechanism was approved, and the future is expected to overcome AD Professor Liu Xueyuan, Shanghai Tenth People's Hospital.
China is a major country with dementia, of which Alzheimer's disease (AD) dementia accounts for 60% to 80%
.
It is estimated that there are currently 10 million AD patients in China, and this number is still rising with the aging of the population.
It is estimated that by 2050, the number of AD patients in China will exceed 40 million
.
Behind this huge number of confirmed diagnoses is the helpless dilemma faced by each individual family
.
The pathogenesis of AD is complex and diverse, and there is no unified conclusion yet.
The β-amyloid hypothesis (Aβ) is currently more widely accepted
.
Amyloid plaques caused by Aβ aggregation are one of the pathological features of AD
.
From a biological point of view, AD is defined as two hallmark protein lesions: Aβ plaques outside neurons and neurofibrillary tangles inside neurons (consisting of hyperphosphorylated tau protein)
.
The pathological changes caused by Aβ in the brain include inflammation, synaptic dysfunction and neurofibrillary tangles
.
Figure 7: Aβ production and deposition play a key role in the pathogenesis of AD.
The unresolved pathogenesis also hinders the development of disease treatment to a certain extent
.
In the past, the clinical treatment of AD was mainly symptomatic treatment, and approved therapeutic drugs can only improve symptoms in a short period of time, and cannot delay the disease process or cure the disease
.
The research and development of new drugs in the AD field was also the worst-hit area.
The failure rate of 244 compounds in 413 trials was as high as 99.
6%
.
The good news is that on June 7, 2021, the good news finally came.
The FDA approved Aducanumab for the treatment of AD patients through the accelerated approval process, and updated the Aducanumab instructions on July 8 to adjust the drug users to AD-derived mild cognitive impairment (MCI) and patients with mild AD
.
Aducanumab is currently the world's first and only human IgG1 anti-Aβ monoclonal antibody that has a clear pathological mechanism of AD approved by the FDA.
It can clear a variety of Aβ aggregates (including oligomers, fibers and plaques) and slow down the progression of the disease
.
In the course of more than ten years of research and development, Aducanumab has accumulated sufficient evidence-based medical evidence
.
The results of phase 1b PRIME study and phase 3 EMERGE and ENGAGE studies all confirmed that after Aducanumab treatment, the pathophysiological biomarker Aβ of AD decreased in a dose- and time-dependent manner
.
Figure 8: Three key studies confirmed that Aducanumab can effectively reduce Aβ deposition.
In addition, the results of the EMERGE study showed that in early AD patients, compared with the placebo group, patients in the high-dose Aducanumab group had the clinical dementia score scale (CDR- SB), Simple Mental State Examination Scale (MMSE), Cognitive Part of AD Rating Scale (ADAS-Cog13), AD Collaborative Research-Abilities of Daily Living Scale for People with Mild Cognitive Impairment (ADCS-ADL-MCI), and The neuropsychiatric scale (NPI-10) has obvious advantages in scoring, further confirming that Aducanumab can alleviate the clinical decline of AD patients within 78 weeks
.
Figure 9: At 78 weeks, the high-dose group significantly delayed the deterioration of cognitive function, vitality, and mental behavior.
In addition, modeling predictions based on the efficacy data of the EMERGE study showed that the addition of Aducanumab treatment may delay the progression of MCI patients compared to standard treatment alone.
The progression of mild, moderate or more severe dementia, in which the progression of MCI to moderate or more severe dementia can be delayed by 2.
58 years (median time)
.
Figure 10: Compared with standard treatment alone, the addition of Aducanumab treatment may delay the progression of dementia in MCI patients.
In terms of safety, during the ENGAGE and EMERGE studies, although the incidence of amyloid imaging abnormalities (ARIA) events in the Aducanumab treatment group was higher than that of placebo It has increased, but most patients have no clinical symptoms
.
The most common ARIA symptoms are headache, confusion, dizziness, and nausea.
Most of them are mild or moderate, and 98% were relieved during the study
.
The approval of Aducanumab not only brings long-lost gospel to AD patients, but also injects a booster for the research of new drugs for AD
.
At present, there are a variety of therapeutic drugs targeting Aβ in the clinical research stage
.
Among them, the research and development of β-secretase 1 (BACE1) inhibitors has invested hugely, but the results of most phase III clinical trials are not optimistic
.
Active immunization (vaccine) and passive immunization (antibodies) to inhibit or eliminate Aβ accumulation and deposition are also current research hotspots.
In terms of active immunotherapy, the fastest progress is CAD106, which has completed phase III clinical trials, but The results have not yet been announced; in passive immunotherapy, the phase II clinical trial of Donanemab has achieved positive results, and the phase III study of BAN2401 is also underway
.
I look forward to more new drugs that will get good news in the future, successfully enter the clinic, and better benefit the majority of AD patients
.
*This article is only used to provide scientific information to medical and health professionals, and does not represent the platform's views
.
On October 29-31, 2021, the 15th China Neurology Forum (CNF 2021) meeting was held in Shanghai
.
Among them, Bojian Biological, as a pioneer in the field of neuroscience, hosted the academic special sessions of the three satellite conferences of multiple sclerosis, spinal muscular atrophy, and Alzheimer's disease to share the latest research progress and clinical diagnosis and treatment experience in the field of neurological diseases
.
The three academic sessions invited Professor Dong Qiang from Huashan Hospital Affiliated to Fudan University and Professor Luo Benyan from the First Affiliated Hospital of Zhejiang University School of Medicine as chairpersons.
Professor Liu Xueyuan from the Tenth People's Hospital of the city gave wonderful reports on related topics
.
Next, the editor will take everyone to briefly review the essence of the academic feast
.
2 major new MS drugs to help patients regain control of their out-of-control lives.
Professor Chen Xiangjun, Huashan Hospital, Fudan University.
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system.
Most people may be relatively uncomfortable with this rare disease.
Relatively unfamiliar, but in fact, in recent years, the global prevalence of MS has shown an increasing trend.
The prevalence in mainland China has increased by nearly 50% between 1990 and 2016
.
As of 2016, the number of MS patients in Mainland China has exceeded 100,000
.
The life of MS patients is out of control.
As the disease progresses and relapses, the patient’s nervous system continues to be damaged.
They may gradually lose their ability to take care of themselves, become blind or even lose their lives.
.
.
The high disability of MS often prevents them from being normal.
According to the 2020 Comprehensive Social Survey of MS Patients in China, as many as 88.
5% of the respondents were unemployed or out of school due to MS; more than 43% of patients cannot take care of themselves completely and need care by others
.
Different from the embarrassing situation that patients with other rare diseases face "it is difficult to seek medical treatment, and it is even more difficult to seek medicine", MS is still a rare disease with medicines available, and the remission period is the key treatment period for MS.
Domestic and foreign guidelines/consensus recommendations Disease modification therapy (DMT) should be initiated as soon as possible during the remission period of MS
.
However, the latest data shows that the current DMT treatment rate in China is only 18%; and the existing DMT drugs in China mainly act on peripheral immunity, so drugs with new mechanisms of action are still needed to deal with MS heterogeneity
.
In April 2021, MS star new drug dimethyl fumarate was approved for marketing in China, bringing new treatment options for MS patients
.
■ Dimethyl fumarate: The world’s most prescribed MS oral DMT drug.
More than 500,000 patients receive treatment.
Dimethyl fumarate has a dual mechanism of action.
It can affect neurons, oligodendrocytes, Microglia and astrocytes have a direct effect to protect the central nervous system; at the same time, dimethyl fumarate regulates the peripheral immune system by regulating the number and function of different types of lymphocytes
.
In terms of efficacy, the comprehensive analysis of the Phase III study DEFINE and CONFIRM studies showed that the annual recurrence rate was reduced by 49% and the risk of disability progression was reduced by 32% after 2 years of dimethyl fumarate treatment
.
In addition, the ENDORSE study with a follow-up of up to 13 years showed that 45% of patients received dimethyl fumarate treatment for 10 years without recurrence, and 77% of patients were not restricted in walking
.
Figure 1: After receiving continuous dimethyl fumarate treatment for 10 years, 45% of patients have no recurrence, and 77% of patients have unrestricted walking.
In terms of safety, the common adverse reactions of dimethyl fumarate are mainly mild to moderate.
, Security is controllable
.
It is particularly worth mentioning that the initial medication of dimethyl fumarate does not require ECG monitoring and genetic testing, and its half-life is short, so there is no need to extend the washout period, and patients can flexibly arrange their birth plans
.
At present, many guidelines/consensus and authoritative journal reviews have listed dimethyl fumarate as the first-line DMT drug.
It is the world's most prescribed oral DMT drug for MS.
More than 500,000 patients have been treated with dimethyl fumarate.
Fully witnessed its efficacy and safety
.
Figure 2: Many guidelines/consensus and authoritative journal reviews have listed dimethyl fumarate as a first-line DMT drug.
One month after dimethyl fumarate was approved in China, MS patients once again ushered in good news, Bojian Another blockbuster new biological drug, Fampridine Sustained-Release Tablets, was also successfully approved.
This time Bojian is targeting MS patients who have difficulty walking
.
Walking ability disorder is one of the key undesirable features of MS.
It can affect patients' physical and mental life and other aspects, including mental health, daily activities, quality of life, and autonomy.
Up to 70% of MS patients with walking difficulties said that walking difficulties are MS The most disturbing aspect
.
However, there is still a lack of effective treatment methods for improving walking disorders suitable for various stages of MS in the clinic.
Taking hormone therapy as an example, although it can alleviate the disease activity in the short term, it has no effect on the progression of long-term disability; while DMT therapy can delay the disease.
Progress, but unable to quickly and effectively symptomatically improve walking disorders
.
The emergence of Fampridine Sustained-Release Tablets made up for this deficiency
.
■ Fampridine sustained-release tablets: the first and currently the only drug approved to improve walking dysfunction in MS patients.
Fampridine sustained-release tablets is a voltage-dependent potassium channel blocker, which can be used as a single agent or combined with DMT Medications, physical therapy and other symptomatic therapies are used for all subtypes of MS patients.
It is the first and currently the only drug approved for the improvement of MS-related walking disorders
.
As of March this year, more than 402,000 patients worldwide have been treated with fampridine sustained-release tablets
.
Fampridine has comprehensive and sufficient randomized controlled and post-marketing studies in many countries
.
The summary analysis of the F203/204 study showed that no matter what type of MS and treatment, Fampridine sustained-release tablets can significantly improve the T25FW (timed 25-foot walking) walking response rate of MS patients
.
Figure 3: Fampridine can significantly improve the patient's T25FW walking response rate regardless of the type of MS and treatment.
In addition, the ENHANCE study shows that starting from the second week, Fampridine sustained-release tablets can significantly improve the patient's 12-item MS walking Table (MSWS-12) score, 24 weeks was significantly better than the placebo group
.
While the efficacy is excellent, the safety of Fampridine Sustained-Release Tablets also performed well.
Most of the adverse events were mild to moderate in severity, and no new adverse events or trends were observed after long-term treatment
.
The successive approvals of dimethyl fumarate and fampridine sustained-release tablets provide MS patients with a comprehensive treatment plan for short-term improvement of walking ability and long-term reduction of disease disability.
It is believed that it will help more patients to regain control of their out-of-control lives.
.
In the era of disease correction treatment, Nosinason is a strong guardian of SMA patients, Professor Jiang Haishan from Southern Medical University Nanfang Hospital shared online that spinal muscular atrophy (SMA) is due to the degenerative disease of spinal motor neurons, leading to skeletal muscle atrophy and general muscle atrophy The hereditary neuromuscular disease was included in China’s "First List of Rare Diseases" in 2018
.
It is estimated that there are currently 850 new patients in China each year, and the total number of patients is about 25,000.
However, the number of registered SMA patients is only about 3,000
.
Based on the age of onset and the maximum motor function achieved, the International SMA Alliance (ISMAC) system classifies SMA from heavier to lighter into 5 subtypes: 0-4
.
Because SMA is the main genetic cause of infant death, the diagnosis and treatment of infant SMA has received more attention, and it is believed that adult SMA patients are only a small part of the disease, and the condition is also lighter
.
In fact, all types of SMA are progressive diseases, and adult SMA patients can be type 1, type 2, type 3, and type 4
.
Figure 4: The composition of adult SMA patients In China, adult SMA patients account for a large proportion of the SMA population, but the diagnosis and treatment of adult SMA patients is more difficult than that of children
.
Although SMA does not lead to faster death of adult patients, as time goes by, the patient's mobility will decline, which will have a great impact on the patient's quality of life.
Therefore, receiving treatment as soon as possible is essential to stabilize the condition and improve the patient's prognosis
.
Unfortunately, there has been no effective treatment for SMA for a long time, and disease management is limited to auxiliary treatment methods such as respiratory support and nutritional support
.
It wasn't until 2016 that the world's first DMT drug Nosinasyn for the cause of SMA came out, the deadlock of "no medicines available" for SMA patients was broken, and the treatment of SMA entered the DMT era
.
Nosinasan is an antisense oligonucleotide (ASO) designed to increase the level of SMN protein by regulating the splicing of the SMN2 gene
.
Nosinasan is administered intrathecally, without crossing the blood-brain barrier, and directly delivers the drug to the cerebrospinal fluid around the spinal cord to give treatment where the disease originated
.
Nosignal was approved to enter China in February 2019, bringing hope to countless families of patients
.
The evidence chain of Nosinathan is complete.
A number of key clinical studies and real-world studies have covered a wide range of SMA patients, including infants, children, and adults of different ages, and have shown consistent efficacy and safety
.
Next, let's take a look at the data from the adult real-world study: The study conducted by Walter et al.
is the first published study to evaluate the efficacy of Noxinazan in the treatment of adult SMA patients
.
The results showed that noxinazan treatment can improve and stabilize the motor function and respiratory function of adult SMA patients, and its overall tolerance is good without serious adverse events
.
Figure 5: Nosinathan treatment can improve and stabilize the motor function and respiratory function of adult SMA patients.
The study conducted by Hagenacker et al.
is the largest real-world study conducted by Nosinathan in adult SMA patients.
There are 173 adult patients
.
The results showed that the patient’s motor function score (HFMSE) continued to increase with the prolonged treatment time, and 96% of the treated patients achieved clinically significant motor function improvement or stability.
The research results have been published in the top journal in the field of neurology, Leaf knife neurology" on
.
Figure 6: 96% of SMA patients treated with Nosinathan have achieved clinically significant improvement or stabilization of motor function.
At present, a number of real-world data have suggested that Nosinathan can stabilize the condition and improve the function of adult SMA patients.
, Change the natural course of disease
.
A wide range of SMA patients worldwide are receiving nosinathan, more than 10,000 cases, of which 33% are adult patients
.
The first treatment plan for a clear pathological mechanism was approved, and the future is expected to overcome AD Professor Liu Xueyuan, Shanghai Tenth People's Hospital.
China is a major country with dementia, of which Alzheimer's disease (AD) dementia accounts for 60% to 80%
.
It is estimated that there are currently 10 million AD patients in China, and this number is still rising with the aging of the population.
It is estimated that by 2050, the number of AD patients in China will exceed 40 million
.
Behind this huge number of confirmed diagnoses is the helpless dilemma faced by each individual family
.
The pathogenesis of AD is complex and diverse, and there is no unified conclusion yet.
The β-amyloid hypothesis (Aβ) is currently more widely accepted
.
Amyloid plaques caused by Aβ aggregation are one of the pathological features of AD
.
From a biological point of view, AD is defined as two hallmark protein lesions: Aβ plaques outside neurons and neurofibrillary tangles inside neurons (consisting of hyperphosphorylated tau protein)
.
The pathological changes caused by Aβ in the brain include inflammation, synaptic dysfunction and neurofibrillary tangles
.
Figure 7: Aβ production and deposition play a key role in the pathogenesis of AD.
The unresolved pathogenesis also hinders the development of disease treatment to a certain extent
.
In the past, the clinical treatment of AD was mainly symptomatic treatment, and approved therapeutic drugs can only improve symptoms in a short period of time, and cannot delay the disease process or cure the disease
.
The research and development of new drugs in the AD field was also the worst-hit area.
The failure rate of 244 compounds in 413 trials was as high as 99.
6%
.
The good news is that on June 7, 2021, the good news finally came.
The FDA approved Aducanumab for the treatment of AD patients through the accelerated approval process, and updated the Aducanumab instructions on July 8 to adjust the drug users to AD-derived mild cognitive impairment (MCI) and patients with mild AD
.
Aducanumab is currently the world's first and only human IgG1 anti-Aβ monoclonal antibody that has a clear pathological mechanism of AD approved by the FDA.
It can clear a variety of Aβ aggregates (including oligomers, fibers and plaques) and slow down the progression of the disease
.
In the course of more than ten years of research and development, Aducanumab has accumulated sufficient evidence-based medical evidence
.
The results of phase 1b PRIME study and phase 3 EMERGE and ENGAGE studies all confirmed that after Aducanumab treatment, the pathophysiological biomarker Aβ of AD decreased in a dose- and time-dependent manner
.
Figure 8: Three key studies confirmed that Aducanumab can effectively reduce Aβ deposition.
In addition, the results of the EMERGE study showed that in early AD patients, compared with the placebo group, patients in the high-dose Aducanumab group had the clinical dementia score scale (CDR- SB), Simple Mental State Examination Scale (MMSE), Cognitive Part of AD Rating Scale (ADAS-Cog13), AD Collaborative Research-Abilities of Daily Living Scale for People with Mild Cognitive Impairment (ADCS-ADL-MCI), and The neuropsychiatric scale (NPI-10) has obvious advantages in scoring, further confirming that Aducanumab can alleviate the clinical decline of AD patients within 78 weeks
.
Figure 9: At 78 weeks, the high-dose group significantly delayed the deterioration of cognitive function, vitality, and mental behavior.
In addition, modeling predictions based on the efficacy data of the EMERGE study showed that the addition of Aducanumab treatment may delay the progression of MCI patients compared to standard treatment alone.
The progression of mild, moderate or more severe dementia, in which the progression of MCI to moderate or more severe dementia can be delayed by 2.
58 years (median time)
.
Figure 10: Compared with standard treatment alone, the addition of Aducanumab treatment may delay the progression of dementia in MCI patients.
In terms of safety, during the ENGAGE and EMERGE studies, although the incidence of amyloid imaging abnormalities (ARIA) events in the Aducanumab treatment group was higher than that of placebo It has increased, but most patients have no clinical symptoms
.
The most common ARIA symptoms are headache, confusion, dizziness, and nausea.
Most of them are mild or moderate, and 98% were relieved during the study
.
The approval of Aducanumab not only brings long-lost gospel to AD patients, but also injects a booster for the research of new drugs for AD
.
At present, there are a variety of therapeutic drugs targeting Aβ in the clinical research stage
.
Among them, the research and development of β-secretase 1 (BACE1) inhibitors has invested hugely, but the results of most phase III clinical trials are not optimistic
.
Active immunization (vaccine) and passive immunization (antibodies) to inhibit or eliminate Aβ accumulation and deposition are also current research hotspots.
In terms of active immunotherapy, the fastest progress is CAD106, which has completed phase III clinical trials, but The results have not yet been announced; in passive immunotherapy, the phase II clinical trial of Donanemab has achieved positive results, and the phase III study of BAN2401 is also underway
.
I look forward to more new drugs that will get good news in the future, successfully enter the clinic, and better benefit the majority of AD patients
.
*This article is only used to provide scientific information to medical and health professionals, and does not represent the platform's views