-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*For medical professionals to read and refer to the results of the Phase II study of Tolebrutinib published in The Lancet, patients with multiple sclerosis welcome the dawn
.
As one of 121 rare diseases included in the "First List of Rare Diseases", multiple sclerosis (MS) is relatively unfamiliar to most people
.
However, as film and television works based on MS patients are on the big screen and new therapeutic drugs continue to be on the market, more and more people are beginning to realize that MS is not far away from us
.
At present, there are about 30,000 MS patients in China [1].
Since MS is a long-term and progressive disabling disease, early diagnosis and treatment of MS should be strengthened in clinical practice
.
In recent years, MS treatment drugs have emerged in endlessly.
There are currently more than 20 kinds of disease modification treatment (DMT) drugs approved at home and abroad [2]; at the same time, there are also a variety of new drugs under development, including Bruton's tyrosine kinase (BTK) inhibitors have attracted much attention
.
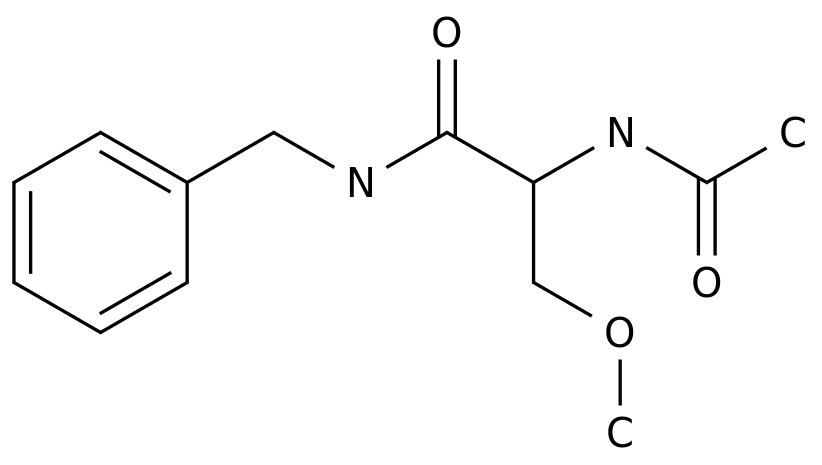
Recently, the top medical journal "The Lancet-Neurology" in the field of neurology published online the results of a phase II study of the oral permeable BTK inhibitor Tolebrutinib in the treatment of relapsed MS [3].
Let us take a look at the data
.
Figure 1: Screenshot of the title of the research titled "The Lancet-Neurology" with its strength.
Tolebrutinib's efficacy and safety are both emphasized.
The study is a 16-week phase IIb, conducted in 40 centers in 10 countries in Europe and North America Randomized, double-blind, placebo-controlled, crossover, dose exploration study
.
The primary efficacy evaluation endpoint of the study is the change in the number of new gadolinium-enhanced lesions in patients after 12 weeks of treatment with Tolebrutinib; secondary endpoints include the number of new or enlarged T2 lesions detected after 12 weeks of treatment, the total number of gadolinium-enhanced lesions, and bad Events and serious adverse events
.
A total of 130 patients with relapsed MS were included in the study
.
The investigator randomly divided the subjects (1:1) that met the inclusion criteria into two cohorts, and then further randomly divided the subjects in each cohort (1:1:1:1:1) into 4 Tolebrutinib dose groups (5, 15, 30 and 60mg/d): Cohort 1 (n=64): First receive Tolebrutinib for 12 weeks, followed by matching placebo (ie tablets with the same appearance) for 4 weeks; Cohort 2 (n=66) : First received 4 weeks of placebo treatment, followed by 12 weeks of Tolebrutinib treatment
.
The results showed that in the 12th week of treatment, Tolebrutinib reduced the number of new gadolinium-enhanced lesions and new or enlarged T2 lesions in MS patients in a dose-dependent manner (P=0.
03; P<0.
0001), of which the 60mg dose group The curative effect is the best.
Compared with placebo, the number of the above two types of lesions after adjustment is relatively reduced by 85% and 89%, respectively
.
At the same time, after 12 weeks of treatment, up to 90% and 87% of the patients in the Tolebrutinib 60mg dose group had no new gadolinium-enhanced lesions and new or enlarged T2 lesions, while in the placebo group during the 4-week treatment period Only 75% and 66% of patients had no new lesions
.
Figure 2: The number of new gadolinium-enhanced lesions after 12 weeks of Tolebrutinib treatment (A: dose-response curve of the number of new gadolinium-enhanced lesions; B: average number of new gadolinium-enhanced lesions in different dose groups) safety, the overall tolerability of Tolebrutinib Good.
The incidence of adverse events between different dose groups is similar.
Common adverse reactions are headache (7%), upper respiratory tract infection (5%) and nasopharyngitis (4%)
.
During the study period, there were no adverse events leading to drug withdrawal or study termination, and no treatment-related deaths occurred
.
This study confirmed that after 12 weeks of treatment, Tolebrutinib can reduce the number of new gadolinium-enhanced lesions in MS patients in a dose-dependent manner.
At the same time, the safety of the drug is good
.
The publication of this research data also injected a booster for exploring the role of Tolebrutinib in the Phase III study, let us look forward to it together
.
The new mechanism of BTK inhibitors is expected to meet the treatment needs of more patients.
The existing MS treatment drugs in the clinic mainly play a role by affecting the peripheral adaptive immunity [3].
Take the previous monoclonal antibody against B lymphocytes as an example.
Dependent cytotoxicity and apoptosis mechanisms consume B cells, but they cannot pass through the blood-brain barrier or lymphatic organs, and cannot enter the central nervous system
.
In order to overcome this limitation of monoclonal antibodies, MS researchers began to focus their research on BTK inhibitors to study their role in the treatment of MS [4]
.
BTK is a member of the non-receptor protein tyrosine kinase family and a key tyrosine kinase in the B cell receptor signaling pathway.
Its abnormal activation is closely related to the occurrence and development of B cell tumors
.
Clinical trials have shown that BTK inhibitors have significant effects on a variety of B-cell tumors, such as chronic lymphocytic leukemia, mantle lymphoma, Waldenstrom's macroglobulinemia, etc.
[5], and development for the treatment of MS is a new attempt
.
In the field of MS diseases, BTK inhibitors can not only act on the peripheral immune system, but also by regulating the adaptive (B cell activation) and innate (CNS astrocytes) immune cells related to neuroinflammation in the brain and spinal cord.
It can cross the blood-brain barrier to act on immune cells that migrate into the brain, and at the same time regulate the astrocytes in the brain related to the process of MS [6-8]
.
Figure 3: BTK is believed to play an important role in B cell and bone marrow/macrophage signal transduction.
The phase I study of Tolebrutinib (also known as SAR442168, PRN2246) shows that Tolebrutinib can be rapidly absorbed after oral administration, and its plasma protein binding The rate is about 93.
5%.
Cerebrospinal fluid penetration can be observed 2 hours after 120mg administration.
In addition to the strong permeability of the blood-brain barrier, even when the dose of Tolebrutinib is as low as 7.
5mg, a higher level of peripheral BTK occupancy can be observed.
Fully confirmed its dual role in the central and peripheral immune system characteristics [9]
.
Most of the DMT drugs approved so far act on peripheral T and B lymphocytes, but studies have shown that both peripheral inflammation and central inflammation play an important role in the progression of MS, and therefore can penetrate the blood-brain barrier and directly act on BTK inhibitors of central inflammation make up for the shortcomings of existing drugs
.
In addition, compared with CD20 monoclonal antibodies that completely deplete B cells, BTK inhibitors can more selectively deplete unwanted B cells by blocking a key enzyme involved in B cell maturation, leaving healthy B cells behind.
[8,10]
.
It can be seen that BTK inhibitors will be expected to meet more needs of MS patients
.
In summary, in the field of tumor treatment, BTK inhibitors have been marketed with a variety of drugs.
Among them, the first-generation BTK inhibitor ibrutinib created the possibility for the chemotherapy-free era of B-cell malignancies; since then, BTK inhibitors have been updated and iterated continuously, and are reducing Continuous progress has been made in toxic and side effects and overcoming drug resistance.
For example, Zebutinib and Obutinib have brought more choices for patients with mantle cell lymphoma, chronic lymphocytic leukemia and small lymphocytic lymphoma
.
Although widely used in the field of oncology, neurologists know very little about BTK inhibitors
.
In fact, the new attempts of "slash youth" BTK inhibitors in the field of MS have also made breakthrough progress.
BTK inhibitors can cross the blood-brain barrier, inhibit central and peripheral inflammation, and make up for the shortcomings of existing MS treatment options.
.
The recent phase II study of the BTK inhibitor Tolebrutinib published by The Lancet confirmed that Tolebrutinib can effectively reduce the number of new gadolinium-enhanced lesions and the number of new or expanded T2 lesions.
The efficacy is dose-dependent; at the same time, it has good safety.
It adds confidence to the phase III study of Tolebrutinib
.
Experts comment that MS is a chronic progressive disease, and there is currently no cure, and patients still need to take medicine for life
.
In recent years, MS treatment drugs have developed rapidly, from interferon, to teriflunomide, fingolimod, sinimod, to CD20 monoclonal antibodies and BTK inhibitors that are currently under development.
The treatment options for patients are Constantly enrich
.
However, most of the existing MS treatment drugs exert therapeutic effects by acting on the peripheral immune system, but have limited effects on inflammation in the central nervous system
.
The emergence of BTK inhibitors has greatly made up for the shortcomings of existing therapeutic drugs.
They can penetrate the blood-brain barrier and affect multiple immune cell signaling pathways related to MS disease in the peripheral and central nervous system
.
Among them, Tolebrutinib is a small-molecule oral drug that can bind to and inhibit BTK in an irreversible manner, and double-inhibit inflammation in the peripheral and central nervous system.
The release of the phase II research data this time provides more for the development of BTK inhibitors.
Confidence and thinking; at the same time, it is worth mentioning that the researchers of the clinical trial pointed out that Tolebrutinib and other BTK inhibitors have different pharmacological characteristics, and they are different in potency, selectivity, and CNS distribution, which are worthy of further evaluation The therapeutic benefits brought by these characteristics
.
It is hoped that with the continuous accumulation of Tolebrutinib research data in the future, Tolebrutinib can be successfully approved, bringing a new mechanism of treatment to MS patients, and allowing more MS patients to return to their normal lives
.
Expert profile Professor Xu Yan, Chief Physician, Professor, and Master's Tutor of Peking Union Medical College Hospital, Deputy Group Leader, Neuroimmunology Group, Chinese Medical Association Neurology Branch, Member of the Chinese Rare Diseases Alliance Nervous System Rare Disease Professional Committee Member, Chinese Medical Doctor Association Neuroimmune Branch Member, Chinese Stroke Society Standing Committee Member of the Neuroimmune Branch, Member of the Neuroimmune Branch of the Chinese Society of Immunology, Member of the Beijing Neurology Association, Neuroinfection and Immunity Professional Committee, Standing Committee Member, Yale University School of Medicine, USA from 2005 to 2007, as a postdoctoral fellow, has been engaged in multiple sclerosis, optic neuromyelitis, optic nerve Clinical and basic research work on central nervous system demyelinating diseases such as inflammation, myelitis, and acute disseminated encephalomyelitis, published more than 60 articles, participated in the compilation of 7 bibliography, independently undertaken or mainly participated in the National Natural Science Foundation of China, Beijing 6 items of the city's natural science fund and college fund
.
References: [1] Survival report of multiple sclerosis patients (2018).
https://mp.
weixin.
qq.
com/s/zA5rArsMnYCJU5AAly_BSg.
[2]Medications_ National Multiple Sclerosis Society.
https:// /Treating-MS/Medications.
[3]Reich DS, Arnold DL, Vermersch P, et al.
Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind , placebo-controlled trial[J].
Lancet Neurol.
2021 Sep;20(9):729-738.
[4]Targeting Bruton Tyrosine Kinase for Multiple Sclerosis Treatment.
https:// bruton-tyrosine-kinase-for-multiple-sclerosis-treatment.
[5]Ji Tingting, Chen Qiuni, Tao Shandong, et al.
Research progress of BTK inhibitors in the treatment of B-cell tumors[J].
Chinese Journal of Experimental Hematology.
2020; 28 (1): 333-338.
[6]THE TAKE ON BTK INHIBITORS: AAN 2021 HAS THE ANSWERS.
https://multiple-sclerosis-research.
org/2021/05/the-take-on-btk-inhibitors-aan -2021-has-the-answers/.
[7]Phase 2b BTKi ('168) Trial Results Dose-finding Study for SAR442168 in Relapsing Multiple Sclerosis.
https:// -COM/Home/common/docs/investors/2020_04_23_BTKi_slides_IR_Call_final_version.
pdf?la=en&hash=971236695489EEF1496B1D0B85805FEF.
[8]Morgan BP, Gommerman JL, Ramaglia V.
An "Outside-In" and "Inside-Out" Consideration of Complement in the Multiple Sclerosis Brain: Lessons From Development and Neurodegenerative Diseases[J].
Front Cell Neurosci.
2021 Jan 7;14:600656.
[9]Smith PF, Redfern A, Shu J, et al.
Phase 1 Clinical Trial of PRN2246 (SAR442168) , a Covalent BTK Inhibitor Demonstrates Safety,CNS Exposure and Therapeutic Levels of BTK Occupancy[J].
ACTRIMS, Feb 28, 2019, P072.
[10]BTK blockers make headway in multiple sclerosis.
https:// This information is for medical and scientific reference only.
Sanofi does not recommend using this product in any way that is inconsistent with the prescription information approved by your country.
.
As one of 121 rare diseases included in the "First List of Rare Diseases", multiple sclerosis (MS) is relatively unfamiliar to most people
.
However, as film and television works based on MS patients are on the big screen and new therapeutic drugs continue to be on the market, more and more people are beginning to realize that MS is not far away from us
.
At present, there are about 30,000 MS patients in China [1].
Since MS is a long-term and progressive disabling disease, early diagnosis and treatment of MS should be strengthened in clinical practice
.
In recent years, MS treatment drugs have emerged in endlessly.
There are currently more than 20 kinds of disease modification treatment (DMT) drugs approved at home and abroad [2]; at the same time, there are also a variety of new drugs under development, including Bruton's tyrosine kinase (BTK) inhibitors have attracted much attention
.
Recently, the top medical journal "The Lancet-Neurology" in the field of neurology published online the results of a phase II study of the oral permeable BTK inhibitor Tolebrutinib in the treatment of relapsed MS [3].
Let us take a look at the data
.
Figure 1: Screenshot of the title of the research titled "The Lancet-Neurology" with its strength.
Tolebrutinib's efficacy and safety are both emphasized.
The study is a 16-week phase IIb, conducted in 40 centers in 10 countries in Europe and North America Randomized, double-blind, placebo-controlled, crossover, dose exploration study
.
The primary efficacy evaluation endpoint of the study is the change in the number of new gadolinium-enhanced lesions in patients after 12 weeks of treatment with Tolebrutinib; secondary endpoints include the number of new or enlarged T2 lesions detected after 12 weeks of treatment, the total number of gadolinium-enhanced lesions, and bad Events and serious adverse events
.
A total of 130 patients with relapsed MS were included in the study
.
The investigator randomly divided the subjects (1:1) that met the inclusion criteria into two cohorts, and then further randomly divided the subjects in each cohort (1:1:1:1:1) into 4 Tolebrutinib dose groups (5, 15, 30 and 60mg/d): Cohort 1 (n=64): First receive Tolebrutinib for 12 weeks, followed by matching placebo (ie tablets with the same appearance) for 4 weeks; Cohort 2 (n=66) : First received 4 weeks of placebo treatment, followed by 12 weeks of Tolebrutinib treatment
.
The results showed that in the 12th week of treatment, Tolebrutinib reduced the number of new gadolinium-enhanced lesions and new or enlarged T2 lesions in MS patients in a dose-dependent manner (P=0.
03; P<0.
0001), of which the 60mg dose group The curative effect is the best.
Compared with placebo, the number of the above two types of lesions after adjustment is relatively reduced by 85% and 89%, respectively
.
At the same time, after 12 weeks of treatment, up to 90% and 87% of the patients in the Tolebrutinib 60mg dose group had no new gadolinium-enhanced lesions and new or enlarged T2 lesions, while in the placebo group during the 4-week treatment period Only 75% and 66% of patients had no new lesions
.
Figure 2: The number of new gadolinium-enhanced lesions after 12 weeks of Tolebrutinib treatment (A: dose-response curve of the number of new gadolinium-enhanced lesions; B: average number of new gadolinium-enhanced lesions in different dose groups) safety, the overall tolerability of Tolebrutinib Good.
The incidence of adverse events between different dose groups is similar.
Common adverse reactions are headache (7%), upper respiratory tract infection (5%) and nasopharyngitis (4%)
.
During the study period, there were no adverse events leading to drug withdrawal or study termination, and no treatment-related deaths occurred
.
This study confirmed that after 12 weeks of treatment, Tolebrutinib can reduce the number of new gadolinium-enhanced lesions in MS patients in a dose-dependent manner.
At the same time, the safety of the drug is good
.
The publication of this research data also injected a booster for exploring the role of Tolebrutinib in the Phase III study, let us look forward to it together
.
The new mechanism of BTK inhibitors is expected to meet the treatment needs of more patients.
The existing MS treatment drugs in the clinic mainly play a role by affecting the peripheral adaptive immunity [3].
Take the previous monoclonal antibody against B lymphocytes as an example.
Dependent cytotoxicity and apoptosis mechanisms consume B cells, but they cannot pass through the blood-brain barrier or lymphatic organs, and cannot enter the central nervous system
.
In order to overcome this limitation of monoclonal antibodies, MS researchers began to focus their research on BTK inhibitors to study their role in the treatment of MS [4]
.
BTK is a member of the non-receptor protein tyrosine kinase family and a key tyrosine kinase in the B cell receptor signaling pathway.
Its abnormal activation is closely related to the occurrence and development of B cell tumors
.
Clinical trials have shown that BTK inhibitors have significant effects on a variety of B-cell tumors, such as chronic lymphocytic leukemia, mantle lymphoma, Waldenstrom's macroglobulinemia, etc.
[5], and development for the treatment of MS is a new attempt
.
In the field of MS diseases, BTK inhibitors can not only act on the peripheral immune system, but also by regulating the adaptive (B cell activation) and innate (CNS astrocytes) immune cells related to neuroinflammation in the brain and spinal cord.
It can cross the blood-brain barrier to act on immune cells that migrate into the brain, and at the same time regulate the astrocytes in the brain related to the process of MS [6-8]
.
Figure 3: BTK is believed to play an important role in B cell and bone marrow/macrophage signal transduction.
The phase I study of Tolebrutinib (also known as SAR442168, PRN2246) shows that Tolebrutinib can be rapidly absorbed after oral administration, and its plasma protein binding The rate is about 93.
5%.
Cerebrospinal fluid penetration can be observed 2 hours after 120mg administration.
In addition to the strong permeability of the blood-brain barrier, even when the dose of Tolebrutinib is as low as 7.
5mg, a higher level of peripheral BTK occupancy can be observed.
Fully confirmed its dual role in the central and peripheral immune system characteristics [9]
.
Most of the DMT drugs approved so far act on peripheral T and B lymphocytes, but studies have shown that both peripheral inflammation and central inflammation play an important role in the progression of MS, and therefore can penetrate the blood-brain barrier and directly act on BTK inhibitors of central inflammation make up for the shortcomings of existing drugs
.
In addition, compared with CD20 monoclonal antibodies that completely deplete B cells, BTK inhibitors can more selectively deplete unwanted B cells by blocking a key enzyme involved in B cell maturation, leaving healthy B cells behind.
[8,10]
.
It can be seen that BTK inhibitors will be expected to meet more needs of MS patients
.
In summary, in the field of tumor treatment, BTK inhibitors have been marketed with a variety of drugs.
Among them, the first-generation BTK inhibitor ibrutinib created the possibility for the chemotherapy-free era of B-cell malignancies; since then, BTK inhibitors have been updated and iterated continuously, and are reducing Continuous progress has been made in toxic and side effects and overcoming drug resistance.
For example, Zebutinib and Obutinib have brought more choices for patients with mantle cell lymphoma, chronic lymphocytic leukemia and small lymphocytic lymphoma
.
Although widely used in the field of oncology, neurologists know very little about BTK inhibitors
.
In fact, the new attempts of "slash youth" BTK inhibitors in the field of MS have also made breakthrough progress.
BTK inhibitors can cross the blood-brain barrier, inhibit central and peripheral inflammation, and make up for the shortcomings of existing MS treatment options.
.
The recent phase II study of the BTK inhibitor Tolebrutinib published by The Lancet confirmed that Tolebrutinib can effectively reduce the number of new gadolinium-enhanced lesions and the number of new or expanded T2 lesions.
The efficacy is dose-dependent; at the same time, it has good safety.
It adds confidence to the phase III study of Tolebrutinib
.
Experts comment that MS is a chronic progressive disease, and there is currently no cure, and patients still need to take medicine for life
.
In recent years, MS treatment drugs have developed rapidly, from interferon, to teriflunomide, fingolimod, sinimod, to CD20 monoclonal antibodies and BTK inhibitors that are currently under development.
The treatment options for patients are Constantly enrich
.
However, most of the existing MS treatment drugs exert therapeutic effects by acting on the peripheral immune system, but have limited effects on inflammation in the central nervous system
.
The emergence of BTK inhibitors has greatly made up for the shortcomings of existing therapeutic drugs.
They can penetrate the blood-brain barrier and affect multiple immune cell signaling pathways related to MS disease in the peripheral and central nervous system
.
Among them, Tolebrutinib is a small-molecule oral drug that can bind to and inhibit BTK in an irreversible manner, and double-inhibit inflammation in the peripheral and central nervous system.
The release of the phase II research data this time provides more for the development of BTK inhibitors.
Confidence and thinking; at the same time, it is worth mentioning that the researchers of the clinical trial pointed out that Tolebrutinib and other BTK inhibitors have different pharmacological characteristics, and they are different in potency, selectivity, and CNS distribution, which are worthy of further evaluation The therapeutic benefits brought by these characteristics
.
It is hoped that with the continuous accumulation of Tolebrutinib research data in the future, Tolebrutinib can be successfully approved, bringing a new mechanism of treatment to MS patients, and allowing more MS patients to return to their normal lives
.
Expert profile Professor Xu Yan, Chief Physician, Professor, and Master's Tutor of Peking Union Medical College Hospital, Deputy Group Leader, Neuroimmunology Group, Chinese Medical Association Neurology Branch, Member of the Chinese Rare Diseases Alliance Nervous System Rare Disease Professional Committee Member, Chinese Medical Doctor Association Neuroimmune Branch Member, Chinese Stroke Society Standing Committee Member of the Neuroimmune Branch, Member of the Neuroimmune Branch of the Chinese Society of Immunology, Member of the Beijing Neurology Association, Neuroinfection and Immunity Professional Committee, Standing Committee Member, Yale University School of Medicine, USA from 2005 to 2007, as a postdoctoral fellow, has been engaged in multiple sclerosis, optic neuromyelitis, optic nerve Clinical and basic research work on central nervous system demyelinating diseases such as inflammation, myelitis, and acute disseminated encephalomyelitis, published more than 60 articles, participated in the compilation of 7 bibliography, independently undertaken or mainly participated in the National Natural Science Foundation of China, Beijing 6 items of the city's natural science fund and college fund
.
References: [1] Survival report of multiple sclerosis patients (2018).
https://mp.
weixin.
qq.
com/s/zA5rArsMnYCJU5AAly_BSg.
[2]Medications_ National Multiple Sclerosis Society.
https:// /Treating-MS/Medications.
[3]Reich DS, Arnold DL, Vermersch P, et al.
Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind , placebo-controlled trial[J].
Lancet Neurol.
2021 Sep;20(9):729-738.
[4]Targeting Bruton Tyrosine Kinase for Multiple Sclerosis Treatment.
https:// bruton-tyrosine-kinase-for-multiple-sclerosis-treatment.
[5]Ji Tingting, Chen Qiuni, Tao Shandong, et al.
Research progress of BTK inhibitors in the treatment of B-cell tumors[J].
Chinese Journal of Experimental Hematology.
2020; 28 (1): 333-338.
[6]THE TAKE ON BTK INHIBITORS: AAN 2021 HAS THE ANSWERS.
https://multiple-sclerosis-research.
org/2021/05/the-take-on-btk-inhibitors-aan -2021-has-the-answers/.
[7]Phase 2b BTKi ('168) Trial Results Dose-finding Study for SAR442168 in Relapsing Multiple Sclerosis.
https:// -COM/Home/common/docs/investors/2020_04_23_BTKi_slides_IR_Call_final_version.
pdf?la=en&hash=971236695489EEF1496B1D0B85805FEF.
[8]Morgan BP, Gommerman JL, Ramaglia V.
An "Outside-In" and "Inside-Out" Consideration of Complement in the Multiple Sclerosis Brain: Lessons From Development and Neurodegenerative Diseases[J].
Front Cell Neurosci.
2021 Jan 7;14:600656.
[9]Smith PF, Redfern A, Shu J, et al.
Phase 1 Clinical Trial of PRN2246 (SAR442168) , a Covalent BTK Inhibitor Demonstrates Safety,CNS Exposure and Therapeutic Levels of BTK Occupancy[J].
ACTRIMS, Feb 28, 2019, P072.
[10]BTK blockers make headway in multiple sclerosis.
https:// This information is for medical and scientific reference only.
Sanofi does not recommend using this product in any way that is inconsistent with the prescription information approved by your country.