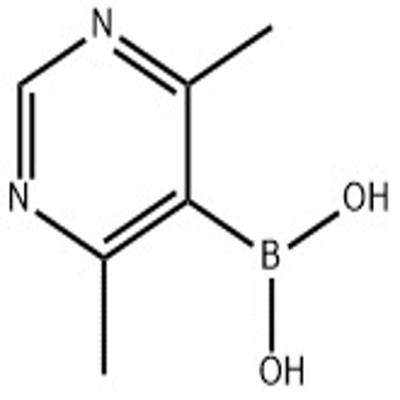
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
The advent of modern immunotherapy has led to changes in the treatment of multiple types of tumors that are limited by a lack of choice of treatment, thus becoming a new milestone in cancer treatment.
ICI has attracted attention for its good efficacy in many solid tumors, including CTLA-4 and its inhibitors, PD-1 and its inhibitors.
subsequent lysovirus, autocytoimmune agents, dual-specific antibodies, step-by-step cell therapy, etc. all showed encouraging results, and the field of cancer treatment was infested.
that while oncology immunotherapy (IO) has become a new "weapon" for cancer treatment, it has gone hand in hand with surgery, chemotherapy and targeted therapy.
, however, IO still has challenges to overcome.
, Dr. Emiliano Calvo of HM Sanchinarro University Hospital in Madrid, Spain, discussed the clinical challenges of immunotherapy and how to optimize them from three different perspectives in a review published in the journal Cancer cell.
: In less than 10 years of saturated development, IO obtained FDA approval for 57 adaptations in 17 solid tumors, 82% of which were PD-1/PD-L1 antibodies.
clinical studies, the number of IO-active drugs being developed has increased from 2,030 to 3,876, an increase of 91% over the past two years, while the number of unique IO targets introduced over the same period has increased by 77%.
, the number of approved drugs has declined in the last two years, mainly in the area of solid tumors.
PD-1/PD-L1 appears to be stable, but the number of active clinical trials has increased 20-fold in the past four years, but this does not match patient recruitment rates, with data showing a 70 percent decrease in the number of patients included in the PD-1/PD-L1 joint trial over the same period.
Overall, it can be argued that there are some saturation signals in IO drug development: relatively few innovative mechanisms of action, including clinical testing, approval stagnation, and saturation of studies that target only certain tumor adaptations and targets.
IO Clinical Challenge Anti-Tumor Activity Although immunosuper inhibitors are unique in their ability to achieve long-term or even complete response, the fact is that most patients still do not benefit from treatment.
, it is imperative to identify reliable markers other than routine radiological assessments to identify tumor biology behavior in patients under IO.
false progressor (PP) is a patient who can benefit from ICI, while hyper-progressor (HP) needs to be detected early in order to be immediately transferred to other treatments.
IO endpoints in clinical trials have shown that the survival curve obtained using ICI drugs is different from conventional treatments.
can be observed in comparative studies with chemotherapy or targeted drugs, IO can show the maximum benefit of the drug at the end of the curve.
, incorrect endpoint selection can cause the experiment to fail.
end point of efficacy in clinical trials requires capturing the longer active timing of these IO drugs, otherwise the benefits of the drug may be underestimated.
total lifetime (OS) is the gold standard in oncology trials, it does not correctly capture the long-term benefits of IO drugs.
a new concept recently introduced - no-treatment lifetime is the ideal end point for cancer treatment because it takes into account long-term survival patients who have no progress and do not require further treatment.
other indicators worth considering include the quality of life test or the time to start the next treatment.
in general, patient benefits should be considered in a more meaningful and comprehensive way, not just progress-free or total lifetimes.
-related adverse events often have different side effects, including different seizure conditions, duration and severity, compared to the use of conventional tumor therapists, and immunotoxicity often requires permanent termination of treatment.
ICI-related adverse events are particularly challenging in complementary treatments, where late and sometimes severe toxicity can lead to permanent damage and these patients could have been cured by surgery.
effective management of IO-related side effects depends on the early identification of symptoms and the rapid initiation of mechanism antidotes, such as steroids or other immunosuppressants, not just palliative drugs.
, it is important to question whether clinical trials can capture these special IO features through the standard Common Terminology Standard for Adverse Events (CTCAE).
addition, it is necessary to establish standardized definitions of objective immune-related adverse events in order to obtain a uniform classification in order to avoid misunderstandings of drug toxicity.
caused by life-threatening ICI may also need to be included in the CTCAE standard, as it can have serious consequences for patients.
dose and schedule strong end-of-drug outcomes combined with clinical responses and major toxicity assessments ultimately determine the recommended dose (RD) for Phase I trials, but most ICI dose-finding studies were unable to identify a maximum to-dosage (MTD) or RD.
further research needs to address whether dose adjustments for individual patients exposed to targeted drugs may result in better outcomes than regular doses.
this will mean cost savings, facilitation for patients, and potentially improved drug activity and toxicity through rigorous treatment index control.
, the optimal length of time patients will need to continue ICI treatment remains unclear.
further randomized trials are needed to explore early ICI aborts and then challenge or biomarker and radiologically driven ICI switching doses to answer this important practical question.
, the authors' findings on immunosuperative inhibitors may help researchers find new breakthroughs that could allow more patients to benefit from cancer immunotherapy.
References: s1. Clinical Challenges of Immune Checkpoint Adderors.




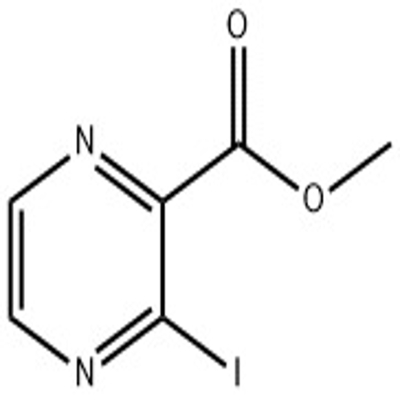
![6-Chloro-1H-pyrazolo[3,4-b]pyrazine](https://file.echemi.com/fileManage/upload/goodpicture/20210820/m20210820163301650.jpg)