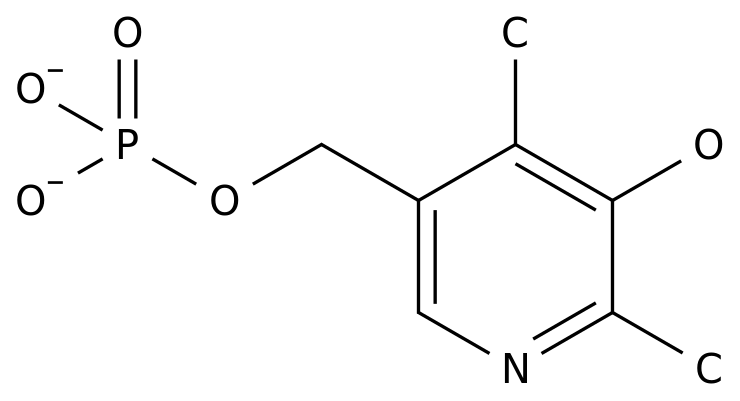
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Medicine Network January 28 reporter 27 self-industry was informed that the State Drug Administration has officially approved China's first combination therapy for the treatment of mild asthma, for adults and adolescents over 12 years of age with mild asthma anti-inflammatory relief treatment, and will provide a full management program for patients with mild, moderate and severe asthma.
studies have shown that 30 to 40 per cent of people with mild asthma may have severe acute seizures.
early anti-inflammatory interventions for mild asthma are important in order to effectively control symptoms and reduce the risk of acute onset.
, as a common chronic respiratory disease, the severity and frequency of asthma vary from person to person.
All asthma patients are at risk of an acute onset, with many patients under-prescribed or under-used anti-inflammatory maintenance therapy and over-reliance on short-acting beta-2-subjected astigmatists (SABA) to relieve treatment, masking the potential for worsening symptoms.
the use of SABA inhalers alone during worsening symptoms not only does not suppress airway inflammation, but also exposes patients to worsening asthma and frequent exposure to oral corticosteroids.
Global Asthma Initiative (GINA) no longer recommends on-demand SABA as the preferred reliant.
the approved combination therapy, called Syngenta (Budine de Fumotro Inhalation Powder Mist II.), is a domestic combination therapy for asthma and chronic obstructive pulmonary disease.
the certificate of approval obtained on the 19th of this month, this combination therapy refers to the inhalation of glucoticoids/long-acting beta-2 subjectors (ICS/LABA) combination therapy.
has previously been approved for initial treatment for all patients with severe asthma to achieve full control of asthma.
as a compound preparation for Budinide (ICS) and Formotro (LABA), Budined Fumotro Inhaled Powder Mist II. Was approved in about 120 countries for the treatment of asthma or chronic obstructive pulmonary disease and was launched in China in 2004.
According to the SYGMA study published in the New England Journal of Medicine, both for patients with poor control after receiving quick-acting inhalation bronchial dilators (SABA) on demand, or for receiving low-dose inhaled corticosteroids or white triene recipient antagonists (LTRA) In patients who were controlled after the on-demand use of quick-acting inhalation bronchial dilators, anti-inflammatory remission therapy of Budined Fumotro Inhaled Powder Mist II. was more effective in follow-up treatment than on-demand treatment of mild asthma with quick-acting beta-2-subject agitators.
Professor Zhou Xin, vice chairman of the 10th Committee of the Chinese Medical Association's Respiratory Credit Association and leader of the Respiratory Science Department of Shanghai First People's Hospital, said that mild asthma is showing an increasing trend, but due to mild symptoms, not enough attention has been paid.
approval of budine de fumotro inhalation powder mist II. New adaptations provides patients and doctors with another treatment plan to meet the clinical needs of a wide range of patients with mild, moderate and severe asthma.
's ®, ® (Budined Fumotro Inhalation Powder Mist II.) was developed by AstraZenecon.
Lei, global executive vice president and president of International Business and China at AstraZeneta, said the future will continue to accelerate the development of innovative treatment options for more Chinese patients, helping to improve the standard of diagnosis and treatment of respiratory diseases and further improve the quality of life of patients.
(Complete)