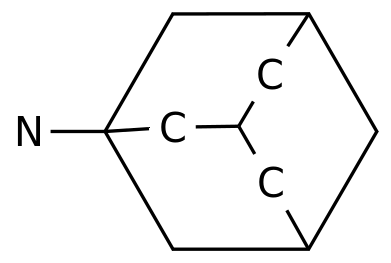
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
October 19, 2020 // -- Eisai recently announced that China's State Drug Administration (NMPA) has accepted a new generation of anti-epileptic drug Fycompa (Wyketai, generic name: perampanel) A new drug application (sNDA) :(1) as a single-drug therapy to treat partial seizure epilepsy≥;
Fycompa was approved in China in September 2019 for complementary treatment of partial seizure epilepsy (accompanied or not accompanied by secondary systemic epilepsy) in patients aged 12 years and older.
estimates that there are about 9 million people with epilepsy in China, and although seizures occur at any age, they are most common in people aged 18 and under and in the elderly.
about 30% of people with epilepsy receive marketable anti-epileptic drugs (AEDs) that do not control seizures, there are significantly unsealed medical needs in this area.
Single-drug treatment partial seizures NDA is based on a subgroup analysis of the safety and effectiveness of patients with single-drug treatment ≥12-year-old partial seizure epilepsy (accompanied by or without secondary systemic epilepsy) based on joint therapeutic clinical studies conducted worldwide (including in the United States, Europe, china≥
In addition, the results of Phase 3 clinical studies (FREEDOM/Study 342) conducted in Japan and Korea were presented as supplementary safety and effective data for untreated 12-74 year olds with or without secondary systemic seizures.
The sNDA of partial seizure epilepsy in pediatric patients is based on the results of Fycompa's Phase III Clinical Study (Study No. 311), which is a complementary treatment for children (under 4 to 12 years of age) with partial seizures or primary systemic hystylinic-tymponic seizure control disorders worldwide.
Fycompa is the first (first-in-class) anti-epileptic drug (AEDs) developed in-house by Aoki, a tablet that is taken once a day.
in the United States and the European Union, fycompa, a new formulation of oral suspension, has been approved for the market.
Fycompa is a highly selective, non-competitive AMPA glutamate-type antagonist.
glutamate is the main neurotransmitter that mediates seizures.
As an AMPA-like antagonist, Fycompa reduces excessive excitability of neurons associated with seizures by targeting the activity of the AMPA-Glutamate after synapses, a mechanism that is different from the anti-epileptic drugs (AEDs) currently on the market.
To date, Fycompa has been approved by more than 70 countries and territories around the world, including Japan, the United States, China and other European and Asian countries, as an ancillary therapy for the treatment of partial seizures (POS, with or without secondary systemic seizures) in patients aged 12 and over.
In addition, Fycompa has been approved by more than 65 countries worldwide, including the United States, Japan and other European and Asian countries, as an ancillary therapy for the treatment of primary full-range PGTC seizures in patients with epilepsy aged 12 years and older.
In Japan, the United States and South Korea, Fycompa has also been approved as a single drug therapy and ancillary therapy for the treatment of partial seizures (with or without secondary systemic seizures) in patients 4 years of age and older.
Fycompa is available in a variety of dosage forms and is available oral once a day before bed.
Japan has approved tablets and fine particulate preparations, oral suspensions and tablets have been approved in the United States and Europe.
, Fycompa has treated more than 300,000 patients worldwide in all adaptations.
currently, Wesser is also conducting a global Phase III clinical study (Study 338) to evaluate Fycompa's treatment for Lenox-Gastaut syndrome-related epilepsy.
addition, Wesser is also developing Fycompa injection preparations.
original source: Add New Drug Applications For Anti-Epileptic Drug Fycompa As Monotherapy For Partial-Onset In China, Pediatrics Appalys For Partial-Onsets Accepted In China