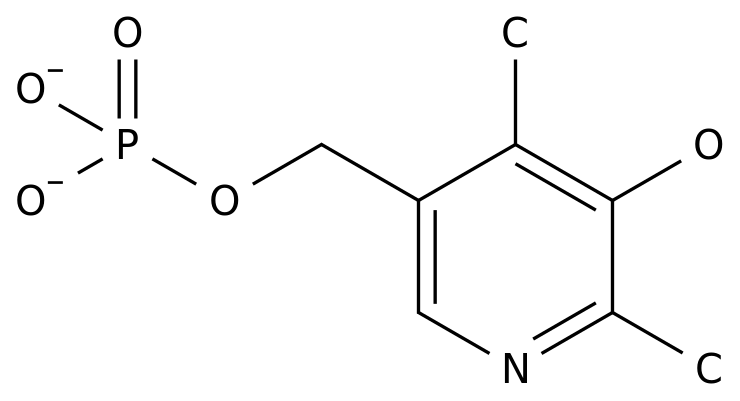
Clinical approval for sofadel for injection, a new drug of class 1.1 in pulo pharmaceutical
-
Last Update: 2020-04-03
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
Puluo pharmaceutical announced on the evening of July 10 that the API sofadel declared by the company was approved for phase I clinical trial Meanwhile, the controlling shareholder affiliated company Hengdian group Jiayuan chemical declared and held the approval document for clinical trial of sofadel preparation and drug Sofadel is a new cardiovascular class 1.1 drug being developed by the company to treat cerebral apoplexy According to the announcement, according to the commitment issued by the controlling shareholder during the 2012 major asset restructuring, all rights of sofadel need to be owned by the listed company pulo pharmaceutical At present, homestead chemical has transferred all relevant technical data of sofadel preparation to homestead pharmaceutical, a holding subsidiary of pulo pharmaceutical According to the data, the current main business structure of Puluo pharmaceutical industry is mainly composed of APIs and intermediates, and the proportion of the preparation revenue is 21.11% The development direction of the company's preparation is focused on anti infection, anti-tumor and cardio cerebrovascular Puluopharma: announcement date of approval of phase I clinical trial of sofadel for injection 2014-07-11 securities code: 000739 securities abbreviation: puluopharma Announcement No.: 2014-33 puluopharma Co., Ltd Announcement on the approval document of phase I clinical trial of sofadel for injection of the company: the company and all members of the board of directors guarantee that the content of information disclosure is true, accurate and complete, and there is no false record, misleading statement or major omission PLO Pharmaceutical Co., Ltd (hereinafter referred to as "the company") recently received the approval document for clinical trials of APIs (approval document No.: 2014l01021) issued by the State Food and Drug Administration (hereinafter referred to as "the State Food and Drug Administration") According to the drug administration law of the people's Republic of China, after the examination of the State Food and drug administration, the raw material drug sofadel declared by the company conforms to the relevant provisions of drug registration and is approved to carry out phase I clinical trial On the same day, the controlling shareholder affiliated company Hengdian group Jiayuan Chemical Co., Ltd (hereinafter referred to as "Jiayuan chemical") declared and held the approval document for clinical trial of sofadel preparation and drug (approval document No.: 2014l01020) Sofadel (hereinafter referred to as "neu2000") is a new cardiovascular chemical drug of class 1.1 being developed by the company to treat cerebral apoplexy According to the commitment issued by the controlling shareholder during the major asset restructuring in 2012, all rights of neu2000 shall be owned by pulo pharmaceutical, a listed company At the time of reorganization, the clinical trial application of neu2000 preparation had been declared, and it was difficult for the applicant to change in the declaration process, and there was great uncertainty about whether to obtain the approval documents Therefore, both parties agreed that the follow-up matters should be managed by PLO pharmaceutical, and their ownership should be changed at zero cost at an appropriate time At present, homestead chemical has transferred all technical data related to neu2000 preparation to homestead pharmaceutical, a subsidiary of pulo pharmaceutical holding company After obtaining the approval documents of phase I clinical trial of neu2000, the company will organize and implement the clinical trial as soon as possible in accordance with the requirements of the national clinical trial The progress and results of follow-up clinical trials are uncertain We sincerely invite investors to make prudent decisions and guard against investment risks It is hereby announced Board of directors of pulo Pharmaceutical Co., Ltd July 10, 2014
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.