Congratulations to Vannosi! The first original new drug was successfully approved for clinical use.
-
Last Update: 2020-07-18
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
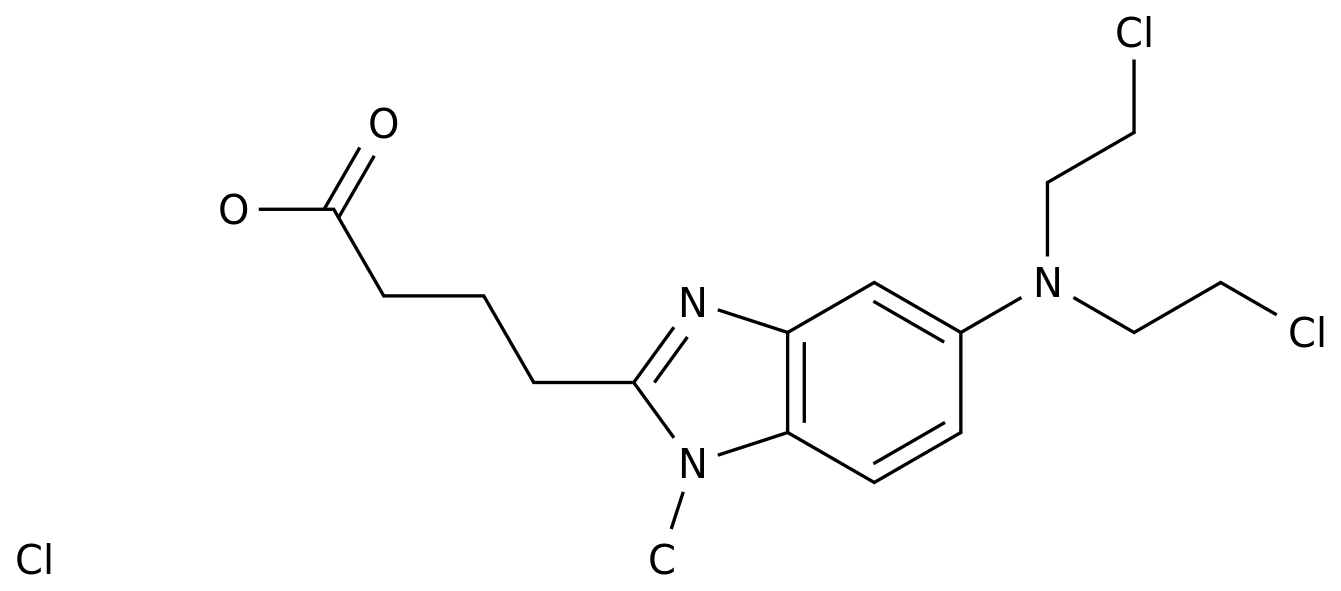
On July 2, 2020, fnx006 tablets, a new class I anti-tumor drug developed by Chengdu van Nux Biomedical Technology Co., Ltd., was approved and issued by the State Drug Administration (nmpa). The company will start phase I clinical trial in China.effect fnx006 is a new drug developed by Chengdu van Nux Biomedical Technology Co., Ltd., which is used to treat advanced malignant solid tumors such as triple negative breast cancer and melanoma.according to the results of preclinical studies, its antitumor activity is better than that of similar drugs.promote all colleagues of Chengdu van Nux under the leadership of president Liu Xiaoyu will do their best to promote the listing of fnx006 products, and provide safe, effective and reasonable therapeutic drugs for patients.Company Profile Chengdu van Nux Biomedical Technology Co., Ltd., located in Chengdu high tech Zone, is a high-tech enterprise specializing in innovative drug research, which was established in March 2017.the company has a number of innovative drug projects in the fields of cancer treatment, antiviral treatment, liver disease and so on, which are in different stages of preclinical research.the team members of the company are from well-known pharmaceutical companies at home and abroad such as GlaxoSmithKline, Lilly and Haizheng, with many years of experience in new drug research and development.Chengdu fanuoxi Biomedical Technology Co., Ltd. is willing to cooperate with people of insight, work hard, blaze new trails, strictly manage, and make due contributions to the medical and health undertakings of all mankind.
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.