-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
"Every small group should not be abandoned"
.
At the beginning of the new year, the news that a child with spinal muscular atrophy, a rare disease in Jiangxi, received medical insurance and welfare drug injections at the Provincial Children's Hospital, swept the circle of friends, and deepened everyone's attention to the rare disease group
.
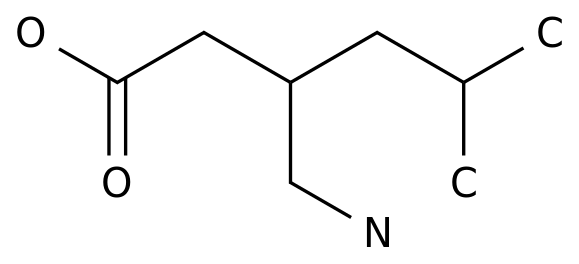
According to the official website of the China National Medical Products Administration (NMPA): Recently, the generic drug teriflunomide developed by Shengshi Tech was officially approved in China
.
The drug's approved indication is relapsing multiple sclerosis, which is the first domestic teriflunomide approved for marketing in China
.
(Image credit: NMPA) Worsening, loss of self-care, blindness and even loss of life
.
According to research by authoritative institutions, multiple sclerosis cannot be cured, and patients have to coexist with the disease
.
Therefore, the earlier the treatment, the better the control of the patient's disease activity and the reduction of irreversible neurological damage
.
Multiple sclerosis (MS) is a lifelong, chronic, progressive disease.
More than 2.
3 million people worldwide suffer from multiple sclerosis, with an incidence rate of about 0.
03%.
There are more than 30,000 patients in China
.
In May 2018, multiple sclerosis was included in China's "First Batch of Rare Disease List"
.
The etiology of the disease stems from the pathological changes of the autoimmune system, causing damage and peeling of the nerve myelin sheath, resulting in damage to the spinal cord, brain and optic nerve functions
.
According to research by authoritative institutions, multiple sclerosis cannot be cured, and patients must live with the disease
.
Therefore, the earlier the treatment, the better the control of the patient's disease activity and the reduction of irreversible neurological damage
.
Teriflunomide developed by Shengshi Tech is an anti-inflammatory immunomodulator that inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme involved in de novo pyrimidine synthesis
.
Studies have shown that the mechanism of action of the drug in the treatment of MS may be related to the reduction of the number of activated lymphocytes in the central nervous system
.
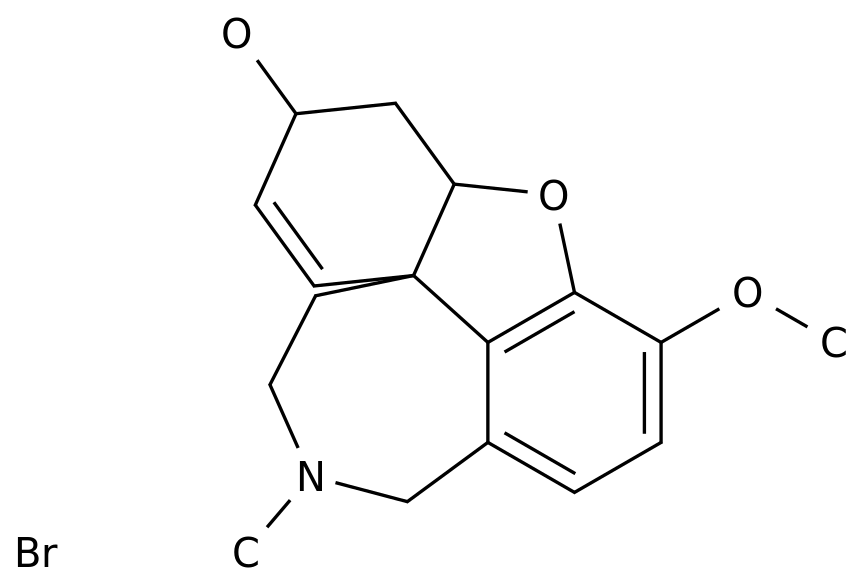
Teriflunomide's original research drug (trade name: Aubagio) was developed by Sanofi and was approved for marketing in China in July 2018 for the treatment of relapsing MS
.
According to Sanofi's 2020 financial report, Aubagio's sales continued to grow to 2.
045 billion euros after its listing, and the approval of pediatric indications is expected to become a new growth driver for Aubagio's sales
.
It is worth noting that in August 2020, the drug was approved in China for the treatment of clinically isolated syndrome (CIS)
.
In fact, rare diseases are not uncommon
.
But without a cure, it is the unspeakable pain of rare disease patients
.
As early as when the FDA approved the drug, Shengshi Taike started the research and development project for the purpose of reducing the recurrence of the patient's disease and delaying the progression of disability, in order to allow patients to use the latest international good drugs as soon as possible
.
In addition to focusing on the research and development of rare disease drugs, the company is also committed to the research and development of hypoglycemic and anti-tumor drugs
.
At present, BioBAY, where Shengshi Tech is located, has 20 new drugs under development that have obtained the "orphan drug certification" of the US FDA.
Enterprise
.
With the launch of Shengshi Taike Teriflunomide, we are one step closer to not giving up the goal of every small group
.
Title picture: The end of Shengshi Taike's official website selected onlookers in the past to "rebel" T cells, which actually helped the tumor to metastasize after chemotherapy! Retext Science: Dietary fiber + probiotics, eating right can help fight cancer, but eating wrong may cause cancer Chinese and American scientists cooperate to discover new therapy against leukemia drug resistance Gratitude 2021, Dream Chasing 2022 Hot Text Inventory 2021 Top 10 Global RNA Biopharmaceutical Companies Medical Immunotherapy | Biosimilars | Vaccines | Drug Resistance | Drug Targets | Healthy Life | Pharmaceutical News | Drug Inventory | Pharmaceutical Technology | Drug Side Effects Basic Research/Translational Medicine Leukemia | Lung Cancer | Gastric Cancer | Colorectal Cancer | Liver Cancer | Breast Cancer | Pancreatic Cancer | Cardiovascular Disease | Printing | Genetic Testing | Single Cell Sequencing | Gene Editing | Assisted Reproduction | Artificial Intelligence | Medical Devices | Telemedicine | WVR Market/Capital IPO | Financing | Cooperation | Funds |
.
At the beginning of the new year, the news that a child with spinal muscular atrophy, a rare disease in Jiangxi, received medical insurance and welfare drug injections at the Provincial Children's Hospital, swept the circle of friends, and deepened everyone's attention to the rare disease group
.
According to the official website of the China National Medical Products Administration (NMPA): Recently, the generic drug teriflunomide developed by Shengshi Tech was officially approved in China
.
The drug's approved indication is relapsing multiple sclerosis, which is the first domestic teriflunomide approved for marketing in China
.
(Image credit: NMPA) Worsening, loss of self-care, blindness and even loss of life
.
According to research by authoritative institutions, multiple sclerosis cannot be cured, and patients have to coexist with the disease
.
Therefore, the earlier the treatment, the better the control of the patient's disease activity and the reduction of irreversible neurological damage
.
Multiple sclerosis (MS) is a lifelong, chronic, progressive disease.
More than 2.
3 million people worldwide suffer from multiple sclerosis, with an incidence rate of about 0.
03%.
There are more than 30,000 patients in China
.
In May 2018, multiple sclerosis was included in China's "First Batch of Rare Disease List"
.
The etiology of the disease stems from the pathological changes of the autoimmune system, causing damage and peeling of the nerve myelin sheath, resulting in damage to the spinal cord, brain and optic nerve functions
.
According to research by authoritative institutions, multiple sclerosis cannot be cured, and patients must live with the disease
.
Therefore, the earlier the treatment, the better the control of the patient's disease activity and the reduction of irreversible neurological damage
.
Teriflunomide developed by Shengshi Tech is an anti-inflammatory immunomodulator that inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme involved in de novo pyrimidine synthesis
.
Studies have shown that the mechanism of action of the drug in the treatment of MS may be related to the reduction of the number of activated lymphocytes in the central nervous system
.
Teriflunomide's original research drug (trade name: Aubagio) was developed by Sanofi and was approved for marketing in China in July 2018 for the treatment of relapsing MS
.
According to Sanofi's 2020 financial report, Aubagio's sales continued to grow to 2.
045 billion euros after its listing, and the approval of pediatric indications is expected to become a new growth driver for Aubagio's sales
.
It is worth noting that in August 2020, the drug was approved in China for the treatment of clinically isolated syndrome (CIS)
.
In fact, rare diseases are not uncommon
.
But without a cure, it is the unspeakable pain of rare disease patients
.
As early as when the FDA approved the drug, Shengshi Taike started the research and development project for the purpose of reducing the recurrence of the patient's disease and delaying the progression of disability, in order to allow patients to use the latest international good drugs as soon as possible
.
In addition to focusing on the research and development of rare disease drugs, the company is also committed to the research and development of hypoglycemic and anti-tumor drugs
.
At present, BioBAY, where Shengshi Tech is located, has 20 new drugs under development that have obtained the "orphan drug certification" of the US FDA.
Enterprise
.
With the launch of Shengshi Taike Teriflunomide, we are one step closer to not giving up the goal of every small group
.
Title picture: The end of Shengshi Taike's official website selected onlookers in the past to "rebel" T cells, which actually helped the tumor to metastasize after chemotherapy! Retext Science: Dietary fiber + probiotics, eating right can help fight cancer, but eating wrong may cause cancer Chinese and American scientists cooperate to discover new therapy against leukemia drug resistance Gratitude 2021, Dream Chasing 2022 Hot Text Inventory 2021 Top 10 Global RNA Biopharmaceutical Companies Medical Immunotherapy | Biosimilars | Vaccines | Drug Resistance | Drug Targets | Healthy Life | Pharmaceutical News | Drug Inventory | Pharmaceutical Technology | Drug Side Effects Basic Research/Translational Medicine Leukemia | Lung Cancer | Gastric Cancer | Colorectal Cancer | Liver Cancer | Breast Cancer | Pancreatic Cancer | Cardiovascular Disease | Printing | Genetic Testing | Single Cell Sequencing | Gene Editing | Assisted Reproduction | Artificial Intelligence | Medical Devices | Telemedicine | WVR Market/Capital IPO | Financing | Cooperation | Funds |