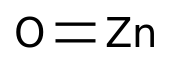Export growth of amino acid products in Taizhou from January to July was in good shape
-
Last Update: 2020-06-29
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
(Communicator Jiang Feijian reporter Zhang Wei) According to the Taizhou Immigration Inspection and Quarantine Bureau statistics, from January to July this year, Taizhou exported a total of 116.86 tons of amino acid products, with a value of 13.2724 million U.SdollarsThe volume and value of exports increased by 12.62 percent and 48.61 percent year-on-year, respectively, showing a good trend of increasing volume pricesrhP
Since May 2007, amino acid products into the legal inspection of goods, Taizhou amino acid products exports from 3.75 million U.Sdollars in 2008 to 21.79 million U.Sdollars in 2014, products are mainly sold to India, the European Union, the Middle East, South America and other more than 30 countries and regions, and gradually become a new export of exports in Taizhou regionrhP
In order to help amino acid products to improve the international market share, Taizhou Entry-Exit Inspection and Quarantine Bureau in the export production enterprises actively implemented the inspection and quarantine supervision model reform"By guiding seven enterprises, including Zhejiang Huahai Pharmaceutical Co., Ltd., to establish and implement risk management plans, we have helped enterprises to further improve the system of conformity assessment of raw material suppliers, acceptance testing of raw materials, risk management of production processes, etc., so that they can improve the quality and safety control level of raw materials, semi-finished products and finished products," said the bureau's animal and plant inspection and quarantine staff"
rhPthe bureau's first-line supervisors also took the initiative to collect the European and American countries in amino acid API products regulatory requirements, and actively promote the United States and the European Union in the pharmaceutical standardsrhP
At the same time, the Bureau actively for enterprises to create a good customs clearance facilitation environmentZhejiang Changming Pharmaceutical Co., Ltdand other seven enterprises to obtain the "declaration and release" of the convenient supervision model, of which four enterprises also enjoy the port inspection and quarantine "green channel" preferential treatmentThese two measures for enterprises to save a large number of port inspection costs, inspection and release speed greatly improved, and reduce the operating costs of enterprisesrhP(Communicator Jiang Feijian reporter Zhang Wei) According to the Taizhou Immigration Inspection and Quarantine Bureau statistics, from January to July this year, Taizhou exported a total of 116.86 tons of amino acid products, the value of 13.2724 million U.SdollarsThe volume and value of exports increased by 12.62 percent and 48.61 percent year-on-year, respectively, showing a good trend of increasing volume pricesrhP
Since May 2007, amino acid products into the legal inspection of goods, Taizhou amino acid products exports from 3.75 million U.Sdollars in 2008 to 21.79 million U.Sdollars in 2014, products are mainly sold to India, the European Union, the Middle East, South America and other more than 30 countries and regions, and gradually become a new export of exports in Taizhou regionrhP
In order to help amino acid products to improve the international market share, Taizhou Entry-Exit Inspection and Quarantine Bureau in the export production enterprises actively implemented the inspection and quarantine supervision model reform "By guiding seven enterprises, including Zhejiang Huahai Pharmaceutical Co., Ltd., to establish and implement risk management plans, we have helped enterprises to further improve the system of conformity assessment of raw material suppliers, acceptance testing of raw materials, risk management of production processes, etc., so that they can improve the quality and safety control level of raw materials, semi-finished products and finished products," said the bureau's animal and plant inspection and quarantine staff "
rhP the bureau's first-line supervisors also took the initiative to collect the European and American countries in amino acid API products regulatory requirements, and actively promote the United States and the European Union in the pharmaceutical standards rhP
At the same time, the Bureau actively for enterprises to create a good customs clearance facilitation environment Zhejiang Changming Pharmaceutical Co., Ltd and other seven enterprises to obtain the "declaration and release" of the convenient supervision model, of which four enterprises also enjoy the port inspection and quarantine "green channel" preferential treatment These two measures for enterprises to save a large number of port inspection costs, inspection and release speed greatly improved, and reduce the operating costs of enterprises rhP
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.