-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
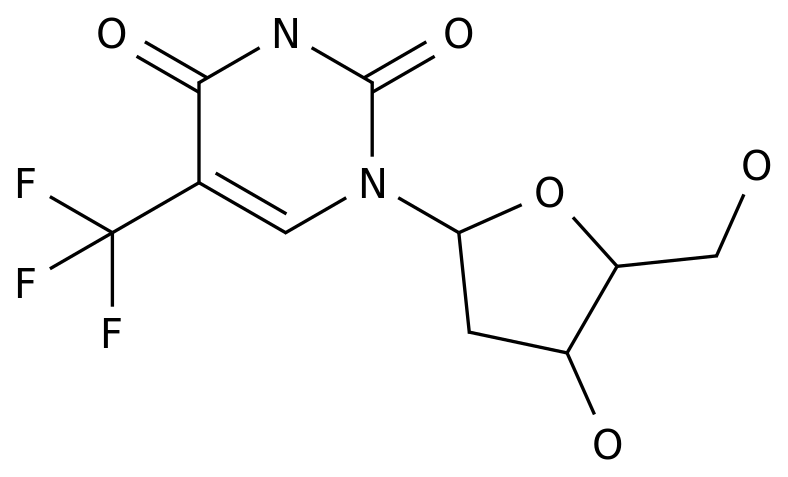
▎Editor of WuXi AppTec's content team On September 15, 2021, Excision BioTherapeutics announced that the US FDA has accepted the IND application for EBT-101
.
EBT-101 is a CRISPR-based candidate therapy designed to functionally cure chronic type 1 human immunodeficiency virus (HIV-1) infection
.
This IND permit enables Excision to initiate the first human phase 1/2 clinical trial of EBT-101 to evaluate the safety, tolerability and efficacy of EBT-101 in HIV-1 infected persons
.
One of the reasons why HIV infection is difficult to cure is that the HIV virus can integrate the DNA into the genome of the host cell after reverse transcription of the RNA genome to produce DNA
.
These integrated DNAs cannot be eliminated by antiviral therapy, they can be transcribed to generate new HIV virus
.
EBT-101 is a therapeutic drug based on CRISPR gene editing in vivo.
It is designed to remove proviral DNA integrated into the genome of human cells.
It is a unique gene therapy
.
In preclinical studies, it has demonstrated the ability to excise HIV proviral DNA in a variety of cell lines, including human primary cells, and a variety of animal models including non-human primates
.
EBT-101 will use adeno-associated virus (AAV) vectors to provide a one-time treatment, aiming to functionally cure HIV infection
.
The research plan uses CRISPR-Cas9 and two guide RNAs to target three sites in the HIV genome, thereby removing most of the HIV genome and minimizing potential virus escape
.
Image source: Excision BioTherapeutics' official website.
Because CRISPR gene editing may have off-target effects, and the repair process that connects the DNA sequence after excision of the proviral DNA may also introduce gene mutations, this clinical trial will initially test the safety of the therapy, and The efficiency of CRISPR to remove proviral DNA
.
Industry experts say that because HIV proviral DNA can be hidden in cells throughout the body, it is difficult for this therapy to completely eliminate them
.
However, it may become part of a curative combination therapy in the future
.
"The FDA's acceptance of the IND application for EBT-101 represents an important milestone for Excision and is the result of our years of dedication to developing a functional cure for HIV-infected patients
.
" said Mr.
Daniel Dornbusch, CEO of Excision, "despite antiviral Drugs can control HIV infection, but patients need to be treated for life, cause side effects, and cannot provide the possibility of functional cure
.
We look forward to launching Phase 1/2 clinical trials later this year
.
"Reference: [1] Excision Receives FDA Clearance of IND for Phase 1/2 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV.
Retrieved September 15, 2021, from https:// -release/2021/09/15/2297456/0/en/Excision-Receives-FDA-Clearance-of-IND-for-Phase-1-2-Trial-of-EBT-101-CRISPR-Based-Therapeutic-for -Treatment-of-HIV.
html Disclaimer: WuXi AppTec's content team focuses on introducing global biomedical health research progress
.
This article is for information exchange purposes only.
The views in the article do not represent WuXi AppTec's position, nor does it represent WuXi AppTec's support or I oppose the views
in the
article .
This article is not a treatment plan recommendation
.
If you need treatment plan guidance, please go to a regular hospital
.
.
EBT-101 is a CRISPR-based candidate therapy designed to functionally cure chronic type 1 human immunodeficiency virus (HIV-1) infection
.
This IND permit enables Excision to initiate the first human phase 1/2 clinical trial of EBT-101 to evaluate the safety, tolerability and efficacy of EBT-101 in HIV-1 infected persons
.
One of the reasons why HIV infection is difficult to cure is that the HIV virus can integrate the DNA into the genome of the host cell after reverse transcription of the RNA genome to produce DNA
.
These integrated DNAs cannot be eliminated by antiviral therapy, they can be transcribed to generate new HIV virus
.
EBT-101 is a therapeutic drug based on CRISPR gene editing in vivo.
It is designed to remove proviral DNA integrated into the genome of human cells.
It is a unique gene therapy
.
In preclinical studies, it has demonstrated the ability to excise HIV proviral DNA in a variety of cell lines, including human primary cells, and a variety of animal models including non-human primates
.
EBT-101 will use adeno-associated virus (AAV) vectors to provide a one-time treatment, aiming to functionally cure HIV infection
.
The research plan uses CRISPR-Cas9 and two guide RNAs to target three sites in the HIV genome, thereby removing most of the HIV genome and minimizing potential virus escape
.
Image source: Excision BioTherapeutics' official website.
Because CRISPR gene editing may have off-target effects, and the repair process that connects the DNA sequence after excision of the proviral DNA may also introduce gene mutations, this clinical trial will initially test the safety of the therapy, and The efficiency of CRISPR to remove proviral DNA
.
Industry experts say that because HIV proviral DNA can be hidden in cells throughout the body, it is difficult for this therapy to completely eliminate them
.
However, it may become part of a curative combination therapy in the future
.
"The FDA's acceptance of the IND application for EBT-101 represents an important milestone for Excision and is the result of our years of dedication to developing a functional cure for HIV-infected patients
.
" said Mr.
Daniel Dornbusch, CEO of Excision, "despite antiviral Drugs can control HIV infection, but patients need to be treated for life, cause side effects, and cannot provide the possibility of functional cure
.
We look forward to launching Phase 1/2 clinical trials later this year
.
"Reference: [1] Excision Receives FDA Clearance of IND for Phase 1/2 Trial of EBT-101 CRISPR-Based Therapeutic for Treatment of HIV.
Retrieved September 15, 2021, from https:// -release/2021/09/15/2297456/0/en/Excision-Receives-FDA-Clearance-of-IND-for-Phase-1-2-Trial-of-EBT-101-CRISPR-Based-Therapeutic-for -Treatment-of-HIV.
html Disclaimer: WuXi AppTec's content team focuses on introducing global biomedical health research progress
.
This article is for information exchange purposes only.
The views in the article do not represent WuXi AppTec's position, nor does it represent WuXi AppTec's support or I oppose the views
in the
article .
This article is not a treatment plan recommendation
.
If you need treatment plan guidance, please go to a regular hospital
.