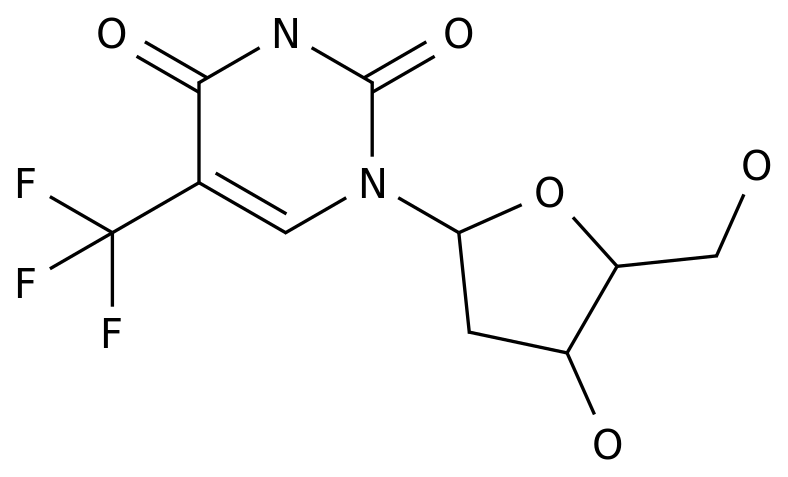
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
▎Editor of WuXi AppTec's content team On January 4, 2022, Gritstone bio announced the second-generation T cell-enhanced self-amplified mRNA (samRNA) new crown vaccine, the latest positive data obtained in a phase 1 clinical trial
.
The trial is evaluating a samRNA vaccine expressing the new coronavirus spike protein and the highly conserved non-spinning protein T cell epitope (TCE) antigen as a booster shot.
It has received two doses of the first-generation AstraZeneca (AstraZeneca) in the past.
The safety, reactogenicity and immunogenicity of the new crown vaccine Vaxzevria (AZD1222) in healthy adults ≥60 years of age
.
The results of the first cohort showed that after being vaccinated with a booster shot of the samRNA vaccine at a dose level of 10 µg, the subjects showed a strong neutralizing antibody response to the new coronavirus spike protein and effectively increased CD8-positive T cells against non-spike protein antigens Level
.
Gritstone bio's samRNA new coronavirus vaccine uses a self-amplifying mRNA system that encodes the spike protein that stimulates the antibody immune response and the TCE that stimulates the immune response of T cells, thereby broadening the immune response to the new coronavirus
.
The self-amplified mRNA system can extend the time of antigen expression and increase the expression level with a smaller dose
.
Image source: The key results of the first cohort (n=10, 10 µg dose level) of Gritstone bio's official website trial are as follows: The new CD8-positive T cell response covers a wide range of non-spike protein epitopes, including Many T cell targets that have been validated in convalescent patients prove the vaccine's potential to prevent the new crown variants
.
Image source: Gritstone bio's official website.
At the same time, the vaccine stimulates a broad and potent neutralizing antibody against the spike protein of the new coronavirus, the level of which is consistent with the published data of the higher dose first-generation mRNA vaccine in a similar clinical setting
.
Image source: Gritstone bio's official website.
In terms of safety, the vaccine is well tolerated, and there are no grade 3/4 adverse events or unexpected reactogenic or safety events
.
"The T cell-enhanced vaccine not only stimulates CD8-positive T cells to produce a strong response to a wide range of viral epitopes, but also promotes a strong neutralizing antibody response to the spike protein
.
We believe this validates the potential of our infectious disease platform
.
" President Gritstone Dr.
Andrew Allen, CEO and co-founder, said, “As we have seen in the Omicron variants, viral surface proteins such as spike proteins are mutating at a high rate, which makes vaccines against spike proteins provide Immunity is easily affected by variants containing many spike protein mutations
.
The new crown vaccine we designed can drive a wide range of CD8-positive T cell immune responses, adding a layer of protection against viruses
.
This innovation can cover a large number of highly conservative viruses Epitopes have the potential to provide stronger clinical protection for current and future new coronavirus variants, and are the first step in the development of pan-coronavirus vaccines
.
"References: [1] Gritstone Announces Positive Clinical Results from First Cohort of a Phase 1 Study (CORAL-BOOST) Evaluating a T Cell-Enhanced Self-Amplifying mRNA (samRNA) Vaccine Against COVID-19.
Retrieved January 4, 2022, from https://ir.
gritstonebio.
com/news-releases/news-release-details/gritstone-announces-positive-clinical-results-first-cohort-phase Disclaimer: WuXi AppTec's content team focuses on introducing global biomedical health Research progress
.
This article is for the purpose of information exchange only.
The views in the article do not represent WuXi AppTec’s position, nor does it mean that WuXi AppTec supports or opposes the views
in the
article .
This article is not a treatment plan recommendation
.
If you need treatment plan guidance, please go to a regular hospital See a doctor
.
.
The trial is evaluating a samRNA vaccine expressing the new coronavirus spike protein and the highly conserved non-spinning protein T cell epitope (TCE) antigen as a booster shot.
It has received two doses of the first-generation AstraZeneca (AstraZeneca) in the past.
The safety, reactogenicity and immunogenicity of the new crown vaccine Vaxzevria (AZD1222) in healthy adults ≥60 years of age
.
The results of the first cohort showed that after being vaccinated with a booster shot of the samRNA vaccine at a dose level of 10 µg, the subjects showed a strong neutralizing antibody response to the new coronavirus spike protein and effectively increased CD8-positive T cells against non-spike protein antigens Level
.
Gritstone bio's samRNA new coronavirus vaccine uses a self-amplifying mRNA system that encodes the spike protein that stimulates the antibody immune response and the TCE that stimulates the immune response of T cells, thereby broadening the immune response to the new coronavirus
.
The self-amplified mRNA system can extend the time of antigen expression and increase the expression level with a smaller dose
.
Image source: The key results of the first cohort (n=10, 10 µg dose level) of Gritstone bio's official website trial are as follows: The new CD8-positive T cell response covers a wide range of non-spike protein epitopes, including Many T cell targets that have been validated in convalescent patients prove the vaccine's potential to prevent the new crown variants
.
Image source: Gritstone bio's official website.
At the same time, the vaccine stimulates a broad and potent neutralizing antibody against the spike protein of the new coronavirus, the level of which is consistent with the published data of the higher dose first-generation mRNA vaccine in a similar clinical setting
.
Image source: Gritstone bio's official website.
In terms of safety, the vaccine is well tolerated, and there are no grade 3/4 adverse events or unexpected reactogenic or safety events
.
"The T cell-enhanced vaccine not only stimulates CD8-positive T cells to produce a strong response to a wide range of viral epitopes, but also promotes a strong neutralizing antibody response to the spike protein
.
We believe this validates the potential of our infectious disease platform
.
" President Gritstone Dr.
Andrew Allen, CEO and co-founder, said, “As we have seen in the Omicron variants, viral surface proteins such as spike proteins are mutating at a high rate, which makes vaccines against spike proteins provide Immunity is easily affected by variants containing many spike protein mutations
.
The new crown vaccine we designed can drive a wide range of CD8-positive T cell immune responses, adding a layer of protection against viruses
.
This innovation can cover a large number of highly conservative viruses Epitopes have the potential to provide stronger clinical protection for current and future new coronavirus variants, and are the first step in the development of pan-coronavirus vaccines
.
"References: [1] Gritstone Announces Positive Clinical Results from First Cohort of a Phase 1 Study (CORAL-BOOST) Evaluating a T Cell-Enhanced Self-Amplifying mRNA (samRNA) Vaccine Against COVID-19.
Retrieved January 4, 2022, from https://ir.
gritstonebio.
com/news-releases/news-release-details/gritstone-announces-positive-clinical-results-first-cohort-phase Disclaimer: WuXi AppTec's content team focuses on introducing global biomedical health Research progress
.
This article is for the purpose of information exchange only.
The views in the article do not represent WuXi AppTec’s position, nor does it mean that WuXi AppTec supports or opposes the views
in the
article .
This article is not a treatment plan recommendation
.
If you need treatment plan guidance, please go to a regular hospital See a doctor
.