FGFR inhibitor is qualified for FDA's prior approval
-
Last Update: 2019-12-03
-
Source: Internet
-
Author: User
Search more information of high quality chemicals, good prices and reliable suppliers, visit
www.echemi.com
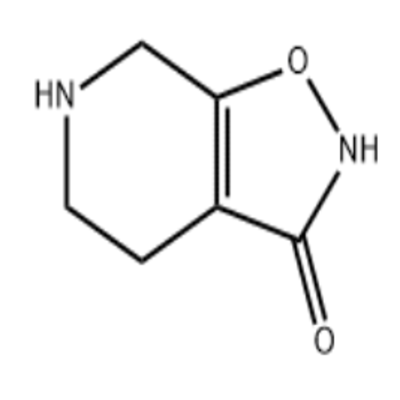
News events Today, Incyte announced that FDA has accepted the listing application of its FGFR inhibitor, pemigatinib, for the second-line treatment of advanced cholangiocarcinoma after FGFR2 fusion or translocation In a phase II clinical trial called light-202, pemigatinib produced a 36% response rate and a median response time of 7.5 months in this population The FDA gives pemigatinib the priority approval qualification, which is usually a priority approval channel to significantly improve the treatment of new drugs without standard therapy, which is 4 months shorter than the standard approval time The PDUFA date for this product is May 30 next year Drug source analysis
This article is an English version of an article which is originally in the Chinese language on echemi.com and is provided for information purposes only.
This website makes no representation or warranty of any kind, either expressed or implied, as to the accuracy, completeness ownership or reliability of
the article or any translations thereof. If you have any concerns or complaints relating to the article, please send an email, providing a detailed
description of the concern or complaint, to
service@echemi.com. A staff member will contact you within 5 working days. Once verified, infringing content
will be removed immediately.