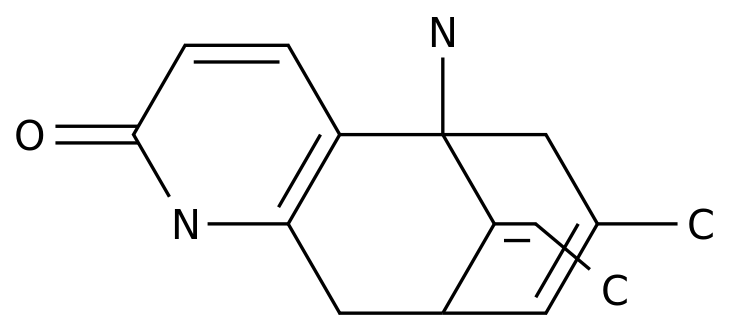
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals to read for reference, more patients will benefit soon! For ischemic stroke, time is the brain.
We have been exploring how to expand the time window of thrombolysis in order to allow more patients to benefit from thrombolysis.
The 2021 International Stroke Conference (ISC) set up a special forum for the topic of expanding the time window for thrombolysis.
Professor Henry Ma from Monash University in Melbourne, Australia, introduced us to the latest research progress of the "infarct core-penumbra mismatch principle".
Exploration: Whether stroke thrombolysis time can be extended.
The IST-3 study published in 2012 [1] (the third international stroke trial, IST-3) evaluated the benefit of extending the thrombolysis time window of acute ischemic stroke to 6 hours after onset And risk.
It is an international, multi-center, randomized controlled clinical trial involving 3,035 patients from 12 countries.
The study showed that thrombolysis within 6 hours of ischemic stroke improved the patient’s functional outcome; In the early stage, patients in the thrombolytic group within 6 hours had a higher risk of symptomatic intracranial hemorrhage and death from cerebrovascular disease.
It should be noted that this is a study conducted without perfusion imaging.
With the development of imaging technology, a number of studies that apply perfusion imaging to the evaluation of thrombolysis in acute ischemic stroke have shown that there is an extended time window for thrombolysis in patients who have already experienced cerebral infarction but have not yet shown infarct tissue on imaging.
Possible.
Challenge: Thrombolysis time window is 9 hours.
In 2019, Professor Henry Ma's team published the ECTEND study in the New England Journal [2] (Figure 1).
The study is a multi-center randomized controlled placebo clinical trial that explores the use of perfusion imaging to guide thrombolysis within 9 hours of acute ischemic stroke.
Infarct core-penumbra mismatch refers to the small size of the infarct core of the patient, but there is a large hypoperfusion area.
The infarct core-penumbra mismatch in this study was defined as: the ratio of the hypoperfusion volume to the infarct core was greater than 1.
2, the absolute volume difference between the two was more than 10 mL, and the infarct core volume was less than 70 mL.
Figure 1 The paper published by Professor Henry Ma's team in the New England Journal [2] Pay attention to the baseline characteristics of the patients in the study.
The average age of the comfort group and the thrombolytic group were 71.
0 years and 73.
7 years, respectively.
The initial median scores of the National Institutes of Health Stroke Scale (NIHSS) in the comfort group and thrombolysis group were 10.
0 points and 12.
0 points, respectively.
A considerable proportion of patients' onset time is within 6-9 hours.
More importantly, the enrolled patients had a large hypoperfusion volume and a relatively small infarct core volume, and approximately 70% of patients had large blood vessel obstruction.
Figure 2 Study baseline data (from Professor Henry Ma's PPT) The study showed that patients undergoing thrombolysis within 4.
5-9 hours had no neurological impairment or minor neurological impairment [90-day modified Rankin score (mRS) 0-1 points] The percentage was higher than that of the placebo group (Figure 3), and the adjusted hazard ratio was 1.
44 [95% confidence interval (CI), 1.
01-2.
06].
There were more cases of symptomatic intracranial hemorrhage in the thrombolytic group [2].
Figure 3 Research results [2] Meta-analysis: A summary of evidence using perfusion imaging to guide thrombolysis.
Next, Professor Henry Ma participated in the meta-analysis of the use of perfusion imaging to guide thrombolysis to 4.
5-9 hours and stroke after wakefulness.
Published in The Lancet Magazine in 2019 [3].
The data for this meta-analysis comes from the aforementioned EXTEND study and two other similar studies.
In this meta-analysis [3], 213 (51%) patients received thrombolytic therapy and 201 (49%) patients received placebo treatment.
The main outcome was a 90-day mRS score of 0-1.
Safety indicators include death and symptomatic intracranial hemorrhage.
The patient's baseline characteristics are similar to those of the EXTEND study, as shown in Figure 4.
Figure 4 Meta-analysis study baseline data (from Professor Henry Ma PPT) The results of the meta-analysis show [3] (Figure 5), the proportion of patients with mRS 0-1 at 90 days is 36% in the thrombolytic group and 9.
5 in the placebo group %, the thrombolysis group had a higher proportion of neurological function without or with small defects, and the adjusted odds ratio was 1.
86 (95%CI: 1.
15-2.
99), p=0.
011.
The proportion of patients with symptomatic intracranial hemorrhage in the thrombolytic group was higher than that in the placebo group [10 (5%) vs.
1 (<1%), adjusted odds ratio was 9.
7, 95% CI: 1.
23-76.
55, p=0.
031] , There was no statistical difference in death cases between the two groups.
The study suggests that with perfusion imaging to guide ischemic stroke (4.
5-9 hours) or stroke after awakening, the neurological outcome of thrombolytic therapy is better than that of placebo, and thrombolytic therapy has a higher risk of bleeding, but it does not weaken thrombolysis.
Overall benefit.
Figure 5 Summary of the meta-analysis study results (from Professor Henry Ma's PPT): It has been included in the recommendation of stroke treatment.
Professor Henry Ma concluded that perfusion imaging can determine the infarct core and ischemic penumbra; perfusion imaging can extend the time of thrombolysis to the occurrence of stroke After 9 hours, it can also be used for stroke after waking up, and the 90-day outcome of thrombolytic therapy is better; this study is reproducible in the real world.
At present, the National Stroke Foundation of Australia has made the following recommendations [4]: For patients with ischemic stroke that may be disabled, in addition to meeting the standard clinical criteria, if they meet the perfusion mismatch criteria, unless the plan is to immediately carry out intravascular thrombosis Resection should be performed within 9 hours after the patient’s last neurological function is intact, and patients with stroke symptoms after waking up should be given intravenous alteplase (dose 0.
9 mg/kg, maximum dose 90 mg) from the time of sleeping (Figure 6). Figure 6 Screenshot of the Australian National Stroke Foundation [4] Speaker reference: [1] IST-3 collaborative group, Sandercock P, Wardlaw JM, et al.
The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke(the third international stroke trial[IST-3]): a randomised controlled trial[published correction appears in Lancet.
2012 Aug 25;380(9843):730].
Lancet.
2012;379(9834):2352- 2363.
[2]Ma H, Campbell BCV, Parsons MW, et al.
Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke.
N Engl J Med.
2019;380(19):1795-1803.
[3] Campbell BCV, Ma H, Ringleb PA, et al.
Extending thrombolysis to 4.
5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data[published correction appears in Lancet.
2020 Jun 20;395(10241):1906].
Lancet.
2019;394(10193):139-147.
[4]https://app.
magicapp.
org/#/guideline/QnoKGn/section/EvvkaE
We have been exploring how to expand the time window of thrombolysis in order to allow more patients to benefit from thrombolysis.
The 2021 International Stroke Conference (ISC) set up a special forum for the topic of expanding the time window for thrombolysis.
Professor Henry Ma from Monash University in Melbourne, Australia, introduced us to the latest research progress of the "infarct core-penumbra mismatch principle".
Exploration: Whether stroke thrombolysis time can be extended.
The IST-3 study published in 2012 [1] (the third international stroke trial, IST-3) evaluated the benefit of extending the thrombolysis time window of acute ischemic stroke to 6 hours after onset And risk.
It is an international, multi-center, randomized controlled clinical trial involving 3,035 patients from 12 countries.
The study showed that thrombolysis within 6 hours of ischemic stroke improved the patient’s functional outcome; In the early stage, patients in the thrombolytic group within 6 hours had a higher risk of symptomatic intracranial hemorrhage and death from cerebrovascular disease.
It should be noted that this is a study conducted without perfusion imaging.
With the development of imaging technology, a number of studies that apply perfusion imaging to the evaluation of thrombolysis in acute ischemic stroke have shown that there is an extended time window for thrombolysis in patients who have already experienced cerebral infarction but have not yet shown infarct tissue on imaging.
Possible.
Challenge: Thrombolysis time window is 9 hours.
In 2019, Professor Henry Ma's team published the ECTEND study in the New England Journal [2] (Figure 1).
The study is a multi-center randomized controlled placebo clinical trial that explores the use of perfusion imaging to guide thrombolysis within 9 hours of acute ischemic stroke.
Infarct core-penumbra mismatch refers to the small size of the infarct core of the patient, but there is a large hypoperfusion area.
The infarct core-penumbra mismatch in this study was defined as: the ratio of the hypoperfusion volume to the infarct core was greater than 1.
2, the absolute volume difference between the two was more than 10 mL, and the infarct core volume was less than 70 mL.
Figure 1 The paper published by Professor Henry Ma's team in the New England Journal [2] Pay attention to the baseline characteristics of the patients in the study.
The average age of the comfort group and the thrombolytic group were 71.
0 years and 73.
7 years, respectively.
The initial median scores of the National Institutes of Health Stroke Scale (NIHSS) in the comfort group and thrombolysis group were 10.
0 points and 12.
0 points, respectively.
A considerable proportion of patients' onset time is within 6-9 hours.
More importantly, the enrolled patients had a large hypoperfusion volume and a relatively small infarct core volume, and approximately 70% of patients had large blood vessel obstruction.
Figure 2 Study baseline data (from Professor Henry Ma's PPT) The study showed that patients undergoing thrombolysis within 4.
5-9 hours had no neurological impairment or minor neurological impairment [90-day modified Rankin score (mRS) 0-1 points] The percentage was higher than that of the placebo group (Figure 3), and the adjusted hazard ratio was 1.
44 [95% confidence interval (CI), 1.
01-2.
06].
There were more cases of symptomatic intracranial hemorrhage in the thrombolytic group [2].
Figure 3 Research results [2] Meta-analysis: A summary of evidence using perfusion imaging to guide thrombolysis.
Next, Professor Henry Ma participated in the meta-analysis of the use of perfusion imaging to guide thrombolysis to 4.
5-9 hours and stroke after wakefulness.
Published in The Lancet Magazine in 2019 [3].
The data for this meta-analysis comes from the aforementioned EXTEND study and two other similar studies.
In this meta-analysis [3], 213 (51%) patients received thrombolytic therapy and 201 (49%) patients received placebo treatment.
The main outcome was a 90-day mRS score of 0-1.
Safety indicators include death and symptomatic intracranial hemorrhage.
The patient's baseline characteristics are similar to those of the EXTEND study, as shown in Figure 4.
Figure 4 Meta-analysis study baseline data (from Professor Henry Ma PPT) The results of the meta-analysis show [3] (Figure 5), the proportion of patients with mRS 0-1 at 90 days is 36% in the thrombolytic group and 9.
5 in the placebo group %, the thrombolysis group had a higher proportion of neurological function without or with small defects, and the adjusted odds ratio was 1.
86 (95%CI: 1.
15-2.
99), p=0.
011.
The proportion of patients with symptomatic intracranial hemorrhage in the thrombolytic group was higher than that in the placebo group [10 (5%) vs.
1 (<1%), adjusted odds ratio was 9.
7, 95% CI: 1.
23-76.
55, p=0.
031] , There was no statistical difference in death cases between the two groups.
The study suggests that with perfusion imaging to guide ischemic stroke (4.
5-9 hours) or stroke after awakening, the neurological outcome of thrombolytic therapy is better than that of placebo, and thrombolytic therapy has a higher risk of bleeding, but it does not weaken thrombolysis.
Overall benefit.
Figure 5 Summary of the meta-analysis study results (from Professor Henry Ma's PPT): It has been included in the recommendation of stroke treatment.
Professor Henry Ma concluded that perfusion imaging can determine the infarct core and ischemic penumbra; perfusion imaging can extend the time of thrombolysis to the occurrence of stroke After 9 hours, it can also be used for stroke after waking up, and the 90-day outcome of thrombolytic therapy is better; this study is reproducible in the real world.
At present, the National Stroke Foundation of Australia has made the following recommendations [4]: For patients with ischemic stroke that may be disabled, in addition to meeting the standard clinical criteria, if they meet the perfusion mismatch criteria, unless the plan is to immediately carry out intravascular thrombosis Resection should be performed within 9 hours after the patient’s last neurological function is intact, and patients with stroke symptoms after waking up should be given intravenous alteplase (dose 0.
9 mg/kg, maximum dose 90 mg) from the time of sleeping (Figure 6). Figure 6 Screenshot of the Australian National Stroke Foundation [4] Speaker reference: [1] IST-3 collaborative group, Sandercock P, Wardlaw JM, et al.
The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke(the third international stroke trial[IST-3]): a randomised controlled trial[published correction appears in Lancet.
2012 Aug 25;380(9843):730].
Lancet.
2012;379(9834):2352- 2363.
[2]Ma H, Campbell BCV, Parsons MW, et al.
Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke.
N Engl J Med.
2019;380(19):1795-1803.
[3] Campbell BCV, Ma H, Ringleb PA, et al.
Extending thrombolysis to 4.
5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data[published correction appears in Lancet.
2020 Jun 20;395(10241):1906].
Lancet.
2019;394(10193):139-147.
[4]https://app.
magicapp.
org/#/guideline/QnoKGn/section/EvvkaE