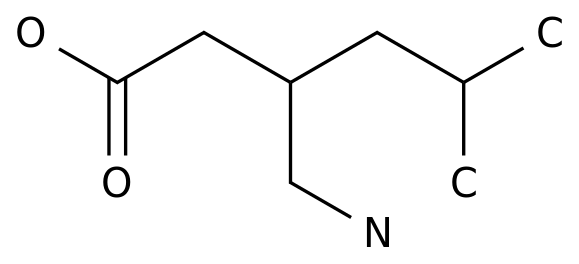
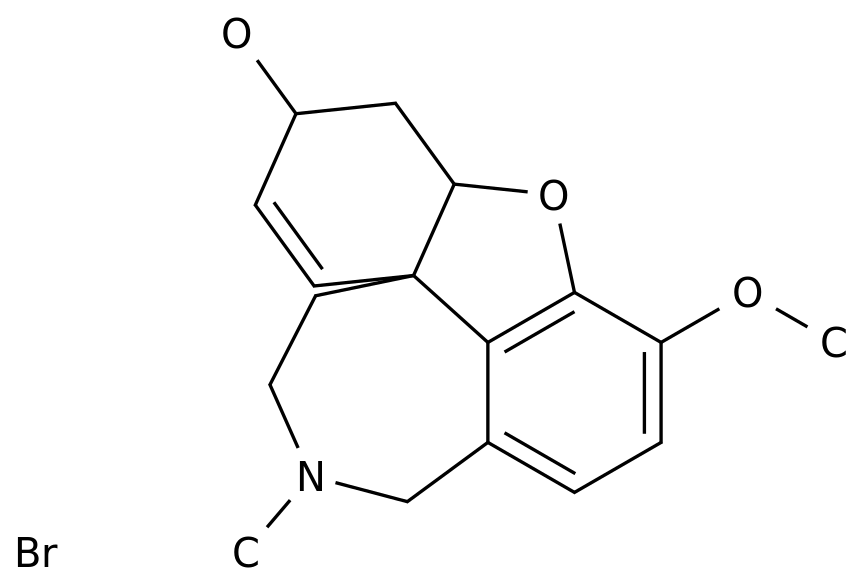
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
*Only for medical professionals' reference.
Effective secondary prevention is an important means to reduce recurrence and death of ischemic stroke or TIA.
Ischemic stroke is the most common type of stroke.
It has the characteristics of high incidence, high disability, high mortality and high recurrence rate.
It is reported that the annual recurrence rate of ischemic stroke in my country is as high as 17.
7%[1, 2].
Transient ischemic attack (TIA) and ischemic stroke are inextricably linked.
A large number of studies have shown that TIA patients have a high risk of stroke in the near future [3].
Effective secondary prevention is an important means to reduce the recurrence and death of ischemic stroke or TIA.
Oral antiplatelet drugs are one of the secondary prevention strategies.
The guidelines recommend that for patients with non-cardiac ischemic stroke or TIA, it is recommended to give oral antiplatelet drugs instead of anticoagulant drugs to prevent stroke and other cardiovascular events.
Aspirin or clopidogrel can be the first antiplatelet drugs[ 2].
So is dual antiplatelet therapy better than monotherapy in preventing ischemic events? At the recently held International Stroke Conference 2021 (International Stroke Conference 2021), four experts from the United States and Spain conducted an in-depth discussion on the anti-platelet therapy strategy of acute ischemic stroke (AIS) or TIA.
Let us work together have a look! The timing of the initial treatment may be an important factor affecting the benefit of double antiplatelets.
The MATCH study is a randomized, double-blind, placebo-controlled trial.
The study included 7599 cases of ischemic stroke or TIA within three months.
The patients were divided into aspirin treatment group and placebo treatment group.
Both groups received clopidogrel routine treatment and followed up for 18 months.
The results showed that combination therapy had no advantage over monotherapy, and major bleeding events increased three times.
Figure 1.
Data from the MATCH study Another randomized, double-blind, placebo-controlled study SP53 also compared the efficacy of aspirin combined with clopidogrel and clopidogrel monotherapy.
The study included 3020 cases of lacunar in 6 months.
For symptomatic patients with infarction, the average follow-up time was 3.
4 years.
The results showed that combination therapy had no advantage over monotherapy, and major bleeding events increased by two times.
Figure 2.
Data from the SP53 study.
Previous studies have shown that the risk of early recurrence after ischemic stroke or TIA is higher.
The risk of recurrence within the first 2 to 7 days is 50%, and the risk of recurrence after 90 days is 5% to 11%.
The MATCH study shows that patients who receive treatment within 7 days can benefit from combination therapy.
Figure 3.
Data from the MATCH study.
Early aspirin-clopidogrel dual antiplatelet therapy for 21 days can bring significant benefits.
The CHANCE study conducted by a Chinese researcher included 5170 cases of mild patients 40 years of age and older who had an onset of within 24 hours.
Patients with stroke or TIA were randomly assigned to the aspirin plus placebo treatment group, or the same aspirin dose plus clopidogrel treatment group.
Patients in the double-antibody treatment group stopped aspirin after 21 days.
The results of the study showed that patients who received both aspirin and clopidogrel had a lower stroke recurrence rate, and the two groups had similar risks of hemorrhagic stroke.
Figure 4.
CHANCE study data The POINT study is an international multicenter randomized trial.
4881 patients with mild ischemic stroke or high-risk TIA within 12 hours of onset were randomly assigned to receive dual antiplatelet therapy or aspirin monotherapy.
Treatment for 90 days.
The results of the study showed that double-antibody treatment significantly reduced the risk of severe ischemic events, but also significantly increased the risk of major bleeding.
Figure 5.
POINT study data.
Aspirin combined with ticagrelor dual antiplatelet therapy can reduce the risk of recurrence, but increase the risk of bleeding.
The SOCRATES study is a multi-center, randomized, double-blind, double-simulated, parallel-group superiority trial, enrolled from around the world There were 13199 patients with acute ischemic stroke or TIA in 674 centers in 33 countries and regions. Patients were randomly assigned to the ticagrelor or aspirin group within 24 hours of the first symptoms of acute ischemic stroke or TIA, and followed up for efficacy for 90 days and safety follow-up for 120 days.
The primary end point of the SOCRATES study was the composite end point from randomization to the first occurrence of stroke (ischemia or hemorrhage), myocardial infarction, and death.
The results of the study showed that the expected effect was not achieved [hazard ratio (HR) = 0.
89, 95% acceptable Confidence interval (CI): 0.
78~1.
01, P=0.
07].
Figure 6.
In terms of the safety of data from the SOCRATES study, ticagrelor is safe for antiplatelet therapy for acute ischemic cerebrovascular events, which is comparable to aspirin (main bleeding P=0.
45).
Figure 7.
Data from the SOCRATES study The THALES study is an international multi-center clinical trial that included more than 11,000 patients with mild acute ischemic stroke or high-risk TIA who started treatment within 24 hours after the onset of symptoms.
Patients were randomly assigned to receive aspirin + ticagrelor (90 mg, 2 times a day) or aspirin (75-150 mg) monotherapy group.
The main efficacy outcome was a composite event of stroke recurrence or death within 30 days.
The results of the study showed that compared with the aspirin single-agent group, the combined events of stroke recurrence or death within 30 days in the ticagrelor combined with aspirin treatment group were significantly reduced.
In terms of safety, consistent with the known safety data of ticagrelor, the bleeding rate in the treatment group increased.
Figure 8.
THALES study data The PRINCE study is a prospective, multi-center, randomized, open-label, positive drug control, blinded clinical trial of outcome evaluation.
It included 675 cases of mild deficiency within 24 hours of onset of 40-80 years of age.
Patients with blood strokes were randomly divided into experimental groups: ticagrelor + aspirin treatment for 21 days, then ticagrelor alone; control group: clopidogrel + aspirin treatment for 21 days, then clopidogrel alone.
The total course of treatment for both groups was 3 months.
In the 7-day and 90-day follow-up, the proportion of patients with high on-treatment platelet reactivity (HOPR, P2Y12reaction unit>208) in the ticagrelor group was all at each follow-up time point.
It was significantly lower than the clopidogrel group (P<0.
001).
Figure 9.
In terms of safety of the PRINCE study data, the ticagrelor group showed more minor bleeding events without intervention (HR=1.
86, P=0.
003) and any bleeding events (HR=1.
65, compared with the clopidogrel group) P=0.
007).
Figure 10.
PRINCE research data reference materials: [1] Guo Wei, Li Dou, Peng Peng.
Chinese expert consensus on emergency first aid for acute ischemic stroke (2018) [J].
Journal of Clinical Emergency Medicine, 2018, 19 (6): 351-359.
[2]Cerebrovascular Diseases Group, Chinese Medical Association Neurology Branch, Chinese Medical Association Neurology Branch.
Guidelines for the Secondary Prevention of Ischemic Stroke and Transient Ischemic Attack in China 2014[J].
Chinese Neurology Journal, 2015, 48 (04): 258-273.
[3] National Health and Family Planning Commission Stroke Prevention and Treatment Engineering Committee.
Guidelines for the Early Diagnosis and Treatment of Transient Ischemic Attack in China (2016).
Effective secondary prevention is an important means to reduce recurrence and death of ischemic stroke or TIA.
Ischemic stroke is the most common type of stroke.
It has the characteristics of high incidence, high disability, high mortality and high recurrence rate.
It is reported that the annual recurrence rate of ischemic stroke in my country is as high as 17.
7%[1, 2].
Transient ischemic attack (TIA) and ischemic stroke are inextricably linked.
A large number of studies have shown that TIA patients have a high risk of stroke in the near future [3].
Effective secondary prevention is an important means to reduce the recurrence and death of ischemic stroke or TIA.
Oral antiplatelet drugs are one of the secondary prevention strategies.
The guidelines recommend that for patients with non-cardiac ischemic stroke or TIA, it is recommended to give oral antiplatelet drugs instead of anticoagulant drugs to prevent stroke and other cardiovascular events.
Aspirin or clopidogrel can be the first antiplatelet drugs[ 2].
So is dual antiplatelet therapy better than monotherapy in preventing ischemic events? At the recently held International Stroke Conference 2021 (International Stroke Conference 2021), four experts from the United States and Spain conducted an in-depth discussion on the anti-platelet therapy strategy of acute ischemic stroke (AIS) or TIA.
Let us work together have a look! The timing of the initial treatment may be an important factor affecting the benefit of double antiplatelets.
The MATCH study is a randomized, double-blind, placebo-controlled trial.
The study included 7599 cases of ischemic stroke or TIA within three months.
The patients were divided into aspirin treatment group and placebo treatment group.
Both groups received clopidogrel routine treatment and followed up for 18 months.
The results showed that combination therapy had no advantage over monotherapy, and major bleeding events increased three times.
Figure 1.
Data from the MATCH study Another randomized, double-blind, placebo-controlled study SP53 also compared the efficacy of aspirin combined with clopidogrel and clopidogrel monotherapy.
The study included 3020 cases of lacunar in 6 months.
For symptomatic patients with infarction, the average follow-up time was 3.
4 years.
The results showed that combination therapy had no advantage over monotherapy, and major bleeding events increased by two times.
Figure 2.
Data from the SP53 study.
Previous studies have shown that the risk of early recurrence after ischemic stroke or TIA is higher.
The risk of recurrence within the first 2 to 7 days is 50%, and the risk of recurrence after 90 days is 5% to 11%.
The MATCH study shows that patients who receive treatment within 7 days can benefit from combination therapy.
Figure 3.
Data from the MATCH study.
Early aspirin-clopidogrel dual antiplatelet therapy for 21 days can bring significant benefits.
The CHANCE study conducted by a Chinese researcher included 5170 cases of mild patients 40 years of age and older who had an onset of within 24 hours.
Patients with stroke or TIA were randomly assigned to the aspirin plus placebo treatment group, or the same aspirin dose plus clopidogrel treatment group.
Patients in the double-antibody treatment group stopped aspirin after 21 days.
The results of the study showed that patients who received both aspirin and clopidogrel had a lower stroke recurrence rate, and the two groups had similar risks of hemorrhagic stroke.
Figure 4.
CHANCE study data The POINT study is an international multicenter randomized trial.
4881 patients with mild ischemic stroke or high-risk TIA within 12 hours of onset were randomly assigned to receive dual antiplatelet therapy or aspirin monotherapy.
Treatment for 90 days.
The results of the study showed that double-antibody treatment significantly reduced the risk of severe ischemic events, but also significantly increased the risk of major bleeding.
Figure 5.
POINT study data.
Aspirin combined with ticagrelor dual antiplatelet therapy can reduce the risk of recurrence, but increase the risk of bleeding.
The SOCRATES study is a multi-center, randomized, double-blind, double-simulated, parallel-group superiority trial, enrolled from around the world There were 13199 patients with acute ischemic stroke or TIA in 674 centers in 33 countries and regions. Patients were randomly assigned to the ticagrelor or aspirin group within 24 hours of the first symptoms of acute ischemic stroke or TIA, and followed up for efficacy for 90 days and safety follow-up for 120 days.
The primary end point of the SOCRATES study was the composite end point from randomization to the first occurrence of stroke (ischemia or hemorrhage), myocardial infarction, and death.
The results of the study showed that the expected effect was not achieved [hazard ratio (HR) = 0.
89, 95% acceptable Confidence interval (CI): 0.
78~1.
01, P=0.
07].
Figure 6.
In terms of the safety of data from the SOCRATES study, ticagrelor is safe for antiplatelet therapy for acute ischemic cerebrovascular events, which is comparable to aspirin (main bleeding P=0.
45).
Figure 7.
Data from the SOCRATES study The THALES study is an international multi-center clinical trial that included more than 11,000 patients with mild acute ischemic stroke or high-risk TIA who started treatment within 24 hours after the onset of symptoms.
Patients were randomly assigned to receive aspirin + ticagrelor (90 mg, 2 times a day) or aspirin (75-150 mg) monotherapy group.
The main efficacy outcome was a composite event of stroke recurrence or death within 30 days.
The results of the study showed that compared with the aspirin single-agent group, the combined events of stroke recurrence or death within 30 days in the ticagrelor combined with aspirin treatment group were significantly reduced.
In terms of safety, consistent with the known safety data of ticagrelor, the bleeding rate in the treatment group increased.
Figure 8.
THALES study data The PRINCE study is a prospective, multi-center, randomized, open-label, positive drug control, blinded clinical trial of outcome evaluation.
It included 675 cases of mild deficiency within 24 hours of onset of 40-80 years of age.
Patients with blood strokes were randomly divided into experimental groups: ticagrelor + aspirin treatment for 21 days, then ticagrelor alone; control group: clopidogrel + aspirin treatment for 21 days, then clopidogrel alone.
The total course of treatment for both groups was 3 months.
In the 7-day and 90-day follow-up, the proportion of patients with high on-treatment platelet reactivity (HOPR, P2Y12reaction unit>208) in the ticagrelor group was all at each follow-up time point.
It was significantly lower than the clopidogrel group (P<0.
001).
Figure 9.
In terms of safety of the PRINCE study data, the ticagrelor group showed more minor bleeding events without intervention (HR=1.
86, P=0.
003) and any bleeding events (HR=1.
65, compared with the clopidogrel group) P=0.
007).
Figure 10.
PRINCE research data reference materials: [1] Guo Wei, Li Dou, Peng Peng.
Chinese expert consensus on emergency first aid for acute ischemic stroke (2018) [J].
Journal of Clinical Emergency Medicine, 2018, 19 (6): 351-359.
[2]Cerebrovascular Diseases Group, Chinese Medical Association Neurology Branch, Chinese Medical Association Neurology Branch.
Guidelines for the Secondary Prevention of Ischemic Stroke and Transient Ischemic Attack in China 2014[J].
Chinese Neurology Journal, 2015, 48 (04): 258-273.
[3] National Health and Family Planning Commission Stroke Prevention and Treatment Engineering Committee.
Guidelines for the Early Diagnosis and Treatment of Transient Ischemic Attack in China (2016).