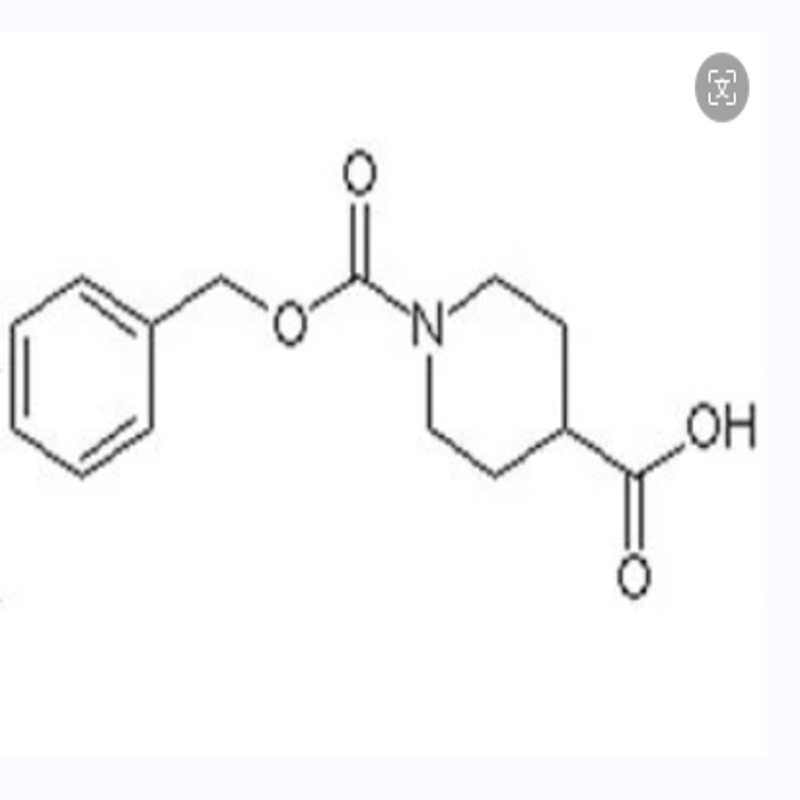
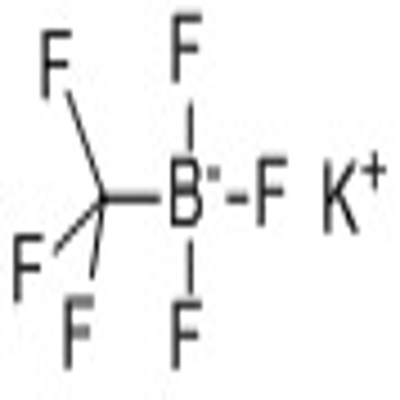
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
June 21, Imcyse, a targeted immunotherapy development company, announced the completion of 35 million euros ($39.5 million) in financing, including 28 million euros ($31.6 million) in round B financing and 6.6 million euros ($7.5 million) in other financing.
In this round of financing, the equity financing of EUR 28 million is led by Life Sciences Partners, FPIM-SFPI and SRIW. In addition, Imcyse received a grant of 4.6 million euros ($5.2 million) from the Walloon region (DGO6) and a bank loan of 2 million euros ($2.3 million) from Belfius.The proceeds of the financing have three uses: first, to advance phase II clinical development trials of Imcyse's modified peptide (Imotops) technology for the treatment of type 1 diabetes;Imcyse is an active-specific immunotherapy company based in Belgium that treats and prevents serious chronic diseases. The company treats autoimmune diseases such as type 1 diabetes, multiple sclerosis and other autoimmune diseases that currently have no cure, primarily by using modified peptides that have the potential to prevent and cure serious chronic autoimmune diseases.Modified peptides can trigger the cleavage of antigen-presenting cells (APC) and the bio-antigen-specific bystander T-cells that are active on their surfaces. APC is induced by antigen-specific cell solubility CD4 T cells. The Imcyse technology platform has demonstrated its ability to specifically eliminate antigen-presenting cells and its own reactive target-specific lymphocytes without affecting other functions of the immune system.At the end of 2017, Imcyse conducted a double-blind, placebo-controlled, incremental clinical study in seven European countries (Belgium, Denmark, France, Germany, Lithuania, Sweden and the United Kingdom). The study was conducted in patients with type 1 type 1 diabetes who had recently developed insulin-dependent diseases. The trial investigated the safety, immune response and effectiveness of modified peptide technology and the results are expected to be published in the summer of 2019. (Arterial mesh)