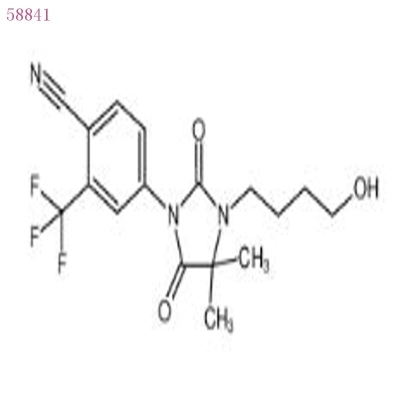
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
Food Partner Network News The website of the Department of Supervision of Processed Food Circulation under the Indonesian Food and DrugAdministration announced on February 9, 2022 that dueimport-related regulations of the Ministry of Trade, the agency stopped issuingimport recommendation letters (SRP) for processed foodof animal)
.
As of November 15, 2021, the import of processed food of animal origin will no longer require an import recommendation letter (SRP) for BPOM
.
Food Partner News Food PartnerNewsFoodManagementImportedAnimals