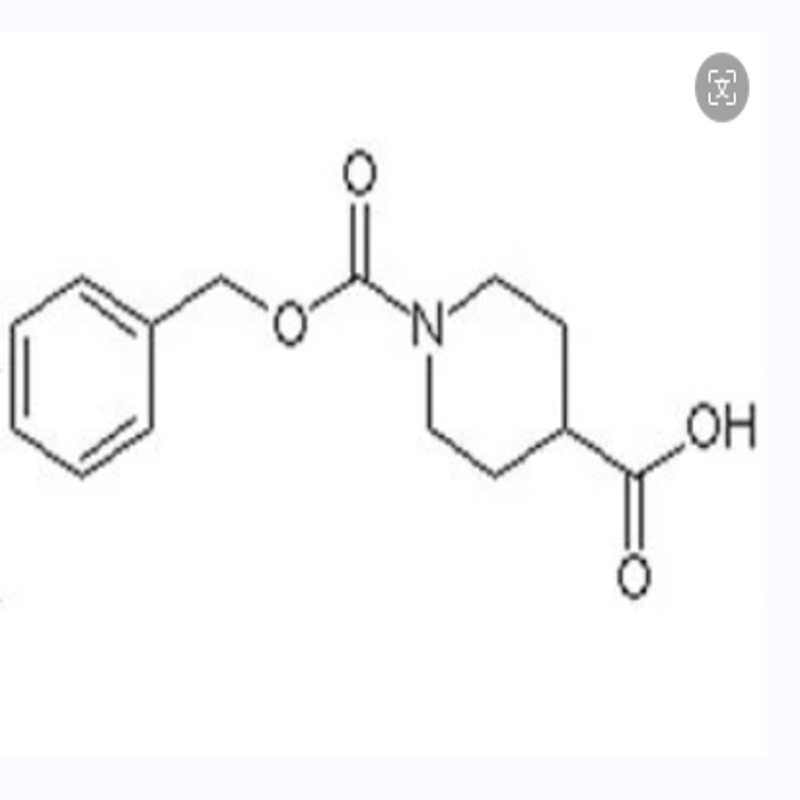
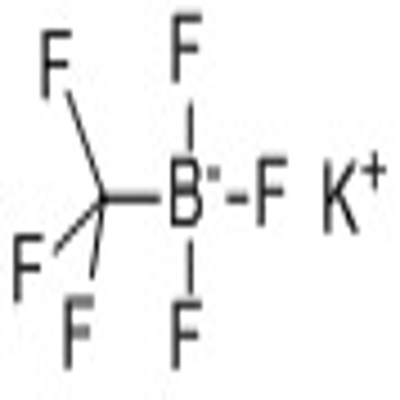
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
"Pharmaceutical Network Market Analysis" Globally, the market for innovative drugs is much larger in terms of revenue than the market for generics and biosimilars, accounting for 67.0% of the total size of the global pharmaceutical market in 2019.
china, innovative drugs also dominate, with data showing that in 2019, the market size of innovative drugs accounted for 56.1 per cent of the total size of the Chinese pharmaceutical market.
innovation can drive China's pharmaceutical field to achieve new leaps.
in the generic drug industry, bid farewell to the era of windfall winners eat, the industry said, we must change the development of ideas, take the road of innovation and development.
" and then take the path of generic drugs is no way out, we must do innovative drugs, have their own native, independent technology to develop.
", for example, said that in foreign generics and innovative drugs are 28 open, generic drug sales accounted for 80%, but the profit is the other way around, generics overall profit of only 20%, the overall profit of innovative drugs 80%.
the future, our country will certainly be in line with international standards, which is a trend.
understood that in recent years, with the development of innovative drugs more and more attention, some enterprises in China have also begun to increase the layout of innovative drugs.
such as Hengrui Pharmaceuticals started with generic drugs, the transformation to innovative drugs, and now medicine is growing rapidly.
understood that Hengrui Pharmaceutical for research and development investment is not stingy.
data show that in the first three quarters of this year, the company committed a total of 3.34 billion yuan in research and development expenses, an increase of 550 million yuan over last year, and the proportion of research and development expenses in revenue increased to 17.2%.
the third quarter of 2020, the research and development cost rate reached 18.27 percent, has reached the international first-line pharmaceutical companies to invest in the proportion.
Up to now, Hengrui Pharmaceutical has developed more than four new drugs 90, of which the country a new class of 4 drugs, two new drugs 13, the company has a number of products square array.
October 20, Hengrui Pharmaceuticals announced that the company's research and development of fluorine Pali capsules and SHR3680 tablets of clinical trials approved, will be conducted in the near future clinical trials, respectively, for pancreatic and prostate cancer, are joint treatment options.
these two products are pancreatic cancer, prostate cancer new drugs, the future is expected to further enrich Hengrui pharmaceutical tumor product line.
forecast, Hengrui pharmaceutical tumor plate will accelerate the release, anesthesia plate, shadow plate and integrated plate flat or slightly increased, the future continued to be optimistic about the rapid growth of innovative drug species.
in recent years, Watson Pharmaceuticals is also in the field of innovative drugs.
In March last year, Watson Pharmaceuticals announced the establishment of a wholly-owned subsidiary in the United States, Watson Pharmaceuticals (USA) Co., Ltd., which focuses on drug research and development, pharmaceutical technology development services and consulting, and in June this year, Watson Pharmaceuticals and Yang Shengyong, Professor of the National Key Laboratory for Biotherapy at Huaxi Hospital of Sichuan University, jointly funded the establishment of Chengdu Ori Pharmaceutical Co., Ltd. to develop innovative drugs for oncology and immunology.
industry pointed out that China still faces many new challenges and opportunities in the research and development of new drugs.
, there is a huge clinical need, especially in the areas of major and rare diseases.
Ii, we should strengthen basic research, take the initiative to connect new breakthroughs in the frontiers of science and technology, and technological research in the frontiers of life sciences and biotechnology, which not only breeds breakthroughs in the discovery of new drugs, but also constantly updates the concept of drug research, creating a new situation and new industry in the pharmaceutical industry.
, fully based on China's national conditions to play Chinese medicine, natural medicine and other advantages.
also strengthen the public relations on drug research and development for major public health emergencies.
understood that from 2016 a number of policy adjustments, China's innovative drug development has opened a new era.
, capital and talent support, so that the development of innovative drugs began to accelerate the stage.
is undeniable that the development of innovative drugs in China is at the right time.
the next three to five years will be a bonus period for a series of policy reforms.
, according to the above-mentioned industry sources, China's new drug research and development momentum is very rapid, the global pre-market new drug research and development contribution from 1-5% in 2016 to 7.8%.
A second tier like Japan and Western European countries, " we predict that by 2030, China's specialty and innovative drugs will occupy the majority of our Chinese pharmaceutical market.
"