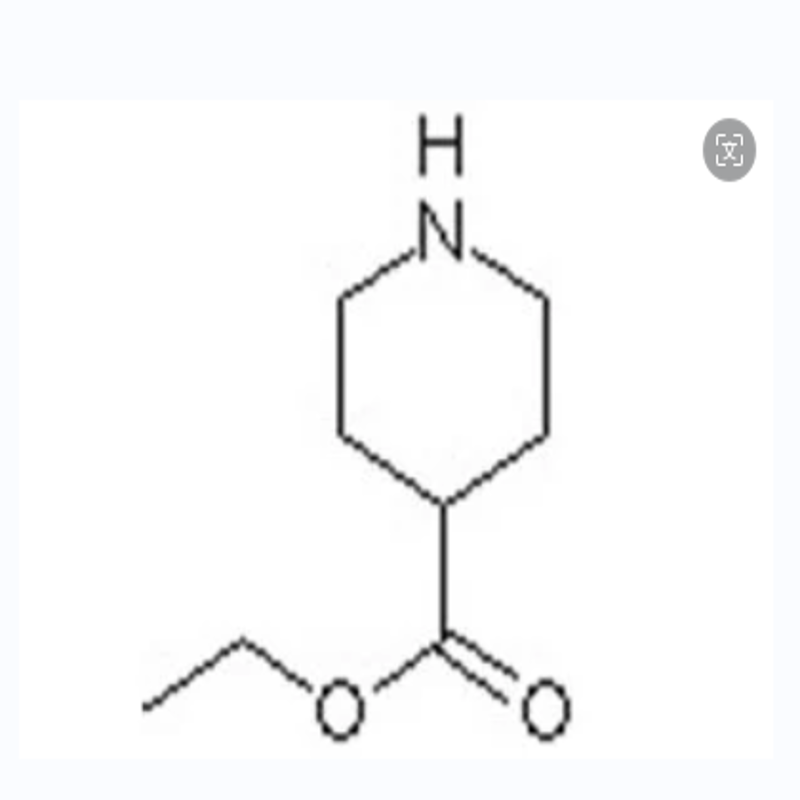
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On October 25, Mirati Therapeutics published two new clinical data on its KRAS G12C selective inhibitor Adagrasib (MRTX849) at the 32nd International Symposium on Molecular Targeting and Cancer Therapy (EORTC-NCI-AACR). It was shown that Adagrasib had an objective remission rate (ORR) of 45% for advanced/metastatic non-small cell lung cancer with KRAS G12C mutation, a disease control rate of 96%, or 17% for colorectal cancer orR with KRAS G12C mutation and a disease control rate of 94%.
In terms of data alone, Adagrasib is really good, after all, Sotorasib (AMG510) just released the latest data on non-small cell lung and colorectal cancer in September, with ORRs of 32.2% and 7.1%, respectively, and disease control rates of 88.1% and 73.8%, respectively.
if Adgrasib has the potential to win Sotorasib, it's important to analyze the information behind these numbers.
, because it is not a head-to-head clinical study, this paper is not a standard sense of good or bad analysis, only for reference.
(Source: NextMed Database) I. Baselines of patients in the group were mostly white, with a median age of 65, current or previous smokers and 89 percent of patients treated with PD-1/L1.
Adagrasib Clinical NSCLC Patient Baseline Profile 2. In Sotorasib's clinical study, 59 NSCLC patients and 42 colorectal cancer patients were included, but the patients were in different dose cohort studies and did not all receive the recommended dose (960 mg). the median age of
is 60, and the proportion of current or previous smokers is comparable to that of Adgrasib, with almost all patients with NSCLC receiving platinum-based chemotherapy and PD-1/L1 treatment, a higher proportion than Adgrasib, with a median line level of 3 for previous systematic anti-cancer treatments.
Sotorasib Clinical Patient Profile II, Effectiveness Data Adagrasib in the NSCLC queue, assessed 51 patients (14 from I./I.b; 37 from Phase II. The patients had objective responses (23/51 patients, 5 of whom had unproven partial reactions and were still receiving treatment), 70% (16/23) had the optimal response to the tumor greater than 40%, and the patient's disease control rate was 96% (49/51).
65 per cent (33/51) of patients were still receiving treatment within 3.6 months of the median follow-up time, and 83 per cent (19/23) of the respondents had not progressed and were still receiving treatment.
Adagrasib's NSCLC effectiveness data, however, Adagrasib's data are for clinically active patient populations with ≥1 assessable lesions, not for all cases included in the study.
Adagrasib's ORR in I./I.b should be 33.3% (6/18) and the disease control rate should be 77.8% (14) if calculated on the basis of all patients included in the study /18). In the I./I.b and II. studies, ORR should be 29.1% (23/79) and disease control 62.0% (49/79).
this doesn't seem to show an advantage over Sotorasib data.
and Sotorasib was in the recommended dose (960 mg) queue, 35.3% (12/34) of patients showed PR and the disease control rate was 91.2% (31/34).
same time as sotorasib effectiveness data, in CRC, Adagrasib single-drug treatment for colorectal cancer with KRAS G12C mutation: ORR 17%, disease control rate 94%.
, the ORR was 12.5 percent and the disease control rate was 70.8 percent, based on patients included in the study.
Adagrasib Clinical CRC and other patient baseline profiles, safety data in 110 patients receiving 600 mg BID, Adagrasib single-drug treatment tolerance is good, any level of adverse reactions (TRAEs) incidence of 85%, the most common (≥20%) adverse reactions are nausea (54%), diarrhea (54%) 51%), vomiting (35%), fatigue (32%) and elevated ALT (20%), the proportion of adverse reactions of level 3 and above was 32%, 1 case of relapsed pneumonia and heart failure of level 5 adverse reactions, 4.5% of patients due to treatment-related adverse events led to drug suspension, adverse reactions led to drug suspension rate of 7.5%.
the proportion of Sotorasib adverse reaction events was higher than the Adagrasib adverse reaction data.
125 patients reported adverse events (96.9%) during treatment, the most common being diarrhoea (38 cases (29.5%)), fatigue (30 cases (23.3%)) and nausea (27 cases (20.9%).
level 3 or higher adverse events (52.7%) were reported in 68 patients.
a total of 73 patients (56.6%) experienced treatment-related adverse events, 2 patients (1.6%) had serious adverse events, and 15 patients (11.6%) reported adverse events related to level 3 or 4 treatment.
treatment-related levels of adverse events include elevated levels of alanine transaminase (4.7%), diarrhea (3.9%), anemia (3.1%), elevation of acetone transaminase (AST) (2.3%), and blood Increased levels of alkaline phosphatase (1.6%), hepatitis (0.8%), lymphocyte count decreased (0.8%), γ-glutamine transirase levels increased (0.8%) and hyponatrexemia (0.8%).
one patient (0.8%) reported an increase in treatment-related level 4 ALT, and one patient (0.8%) was interrupted by an increase in treatment-related levels of level 3 ALT and AST.
Sotorasib adverse reaction data, both Adagrasib and Sotarasib have level 5 adverse events, but there is little difference in the probability of drug suspension due to adverse reactions (7.5% vs 7.0%).
Overall, in terms of effectiveness, Adagrasib had a total ORR of 29.1% (23/79 for all patients in the study, the same dose of the drug), sotorasib in the recommended dose (960 mg) queue, 35.3% (12/34) of patients showed PR, and Sotorasib had a higher disease control rate than Adagrasib;
Of course, the above data are not the final clinical data of the two drugs, the clinical sample size is small, not to be a head-to-head clinical comparison, only in the current data to spy on the potential clinical effectiveness of the two KRAS G12C inhibitors differences.
: #1 KRASG12C Adsion with Sotorasib inAdvanced Solid Tumors, N Engl J Med, September 20, 2020 s2 Mirati, October 25, 2020, InvestorEvent at EORTC-NCI-AACR Virtual Conference