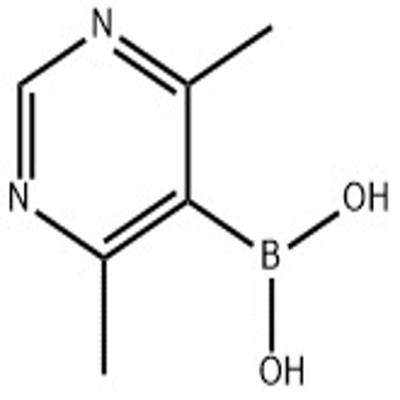
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On the evening of November 20th, the National Health Care Administration issued three consecutive heavyweight documents to explain and arrange two documents that had previously attracted much industry attention.
1, pharmaceutical companies credit rating, the rules came to this "pharmaceutical price and credit evaluation of the operating norms (2020 version)" and "pharmaceutical prices and credit rating of the discretionary benchmark (2020 version)" officially released, meaning that the rules have been issued, from the implementation of the policy is not far from the landing.
The document clearly states that in order to promote the fair and orderly development of pharmaceutical prices and credit rating, unify the scale of credit rating, strengthen the coordination and balance of credit rating work between regions, formulate discretionary benchmarks and operating norms, and if the price or marketing behavior of pharmaceutical enterprises conforms to one of the following circumstances, the failure rating shall be assessed as "general": according to the facts of the case as determined by the court decision or the administrative punishment of the relevant law enforcement department, nearly three years (excluding 20 years) Prior to August 28, 20 years ago, the same as 20) in the province, all kinds of medical institutions at all levels, centralized procurement agencies and their staff have been given kickbacks and other medical commercial bribery, the same case accumulated bribe amount of more than 10,000 yuan (including 10,000 yuan, the same between), less than 150,000 yuan (excluding 150,000 yuan, the same), or a single bribe amount of more than 10,000 yuan, less than 100,000 yuan.
it is worth noting that the commercial bribery of pharmaceuticals called in this paragraph includes commercial bribery between pharmaceutical companies, as well as commercial bribery between its employees (including employment relationships, the same between them) or distribution enterprises with principal-agent relationships.
Medium: According to the facts of the case determined by the court judgment or the administrative punishment of the relevant law enforcement departments, in the past three years, various medical institutions at all levels, centralized procurement agencies and their staff have been given kickbacks and other medical commercial bribery, the cumulative amount of bribes in the same case is more than 150,000 yuan, less than 500,000 yuan, or a single bribe amount of more than 100,000 yuan, less than 300,000 yuan.
case of false VAT invoices investigated and dealt with by the tax departments of the region belongs to the party that obtained the false VAT invoices, and the total amount of the price tax involved in the case totals more than 100,000 yuan, less than 1 million yuan.
Serious: According to the facts of the case determined by the court judgment or the administrative punishment of the relevant law enforcement departments, in the past three years, various medical institutions at all levels, centralized procurement agencies and their staffs have been given kickbacks and other commercial bribery of medicine, the cumulative amount of bribes in the same case is more than 500,000 yuan, less than 2 million yuan, or a single bribe amount of more than 300,000 yuan, less than 2 million yuan.
case of false VAT invoice investigated and dealt with by the tax department of the region belongs to the party that obtained the false VAT invoice, and the total amount of the price tax involved is more than 1 million yuan and less than 10 million yuan.
Is particularly serious: according to the court's judgment or the relevant law enforcement departments administrative punishment identified the facts of the case, in the past three years in the province, all kinds of medical institutions at all levels, centralized procurement agencies and their staff have been given kickbacks and other medical commercial bribery, the same case accumulated or a single bribe amount of more than 2 million yuan.
case of false VAT invoice investigated and dealt with by the tax department of the region belongs to the party that obtained the false VAT invoice, and the total amount of the value tax involved is more than 10 million yuan.
2. How to define the scope of credit evaluation and a series of evaluation processes in the documents issued by the State Administration of Medical Security, and again refine the specific operating norms: pharmaceutical enterprises pricing, marketing, bidding, performance process in the implementation of laws and regulations prohibited, contrary to good faith and fair competition for improper benefits, such as in the purchase and sale of medicine to give kickbacks or other improper benefits (hereinafter referred to as "pharmaceutical commercial bribery"), the implementation of monopoly, price and tax-related violations, malicious violations of contract agreements.
evaluation includes: "In the purchase and sale of medicines, all kinds of medical institutions at all levels, centralized procurement agencies and their staff rebates or other improper benefits."
"Obtain a false VAT invoice (except for a false VAT invoice in good faith)."
"due to their own or related enterprises to implement monopoly agreements, abuse of market dominance, etc. were punished according to law, do not take the initiative to correct the unfair high prices of the products involved."
"fabricate, spread price increase information, raise prices, promote excessive price increases and other violations of the Price Law."
"Pharmaceutical enterprises due to improper price behavior, by the pharmaceutical price authorities letter inquiry, investigation, interview, admonishment, inspection, etc., push, refuse, can not fully explain the reasons or make false commitments."
is "bidding at below-cost bids, bidding by fraud, bid rigging, abuse of market dominance, etc., to disrupt the centralized procurement order".
"refusal to perform commitments, refusal to perform purchase and sale or distribution contracts without justification."
addition, the document clearly, the implementation of corporate commitments.
pharmaceutical enterprises to participate in or be entrusted to participate in the centralized belt procurement of pharmaceutical and medical supplies, platform hanging network (including record procurement), should make a written commitment to keep faith.
undertakes to be liable for breach of trust if its employees (including employment relations, as well as labor dispatch, purchase services, entrustment agents, etc., hereinafter referred to as employees), or distribution enterprises with principal-agent relationships (hereinafter referred to as entrusted agent enterprises) commit breach of trust to enable their own medicines or medical supplies to obtain or increase trading opportunities and competitive advantages.
this means that submitting a commitment is a prerequisite for entering the hospital and requires pharmaceutical representatives, agents, and pharmaceutical companies to make credit bindings, and that there will be no later claims that "pharmaceutical bribery is an act of a representative or an individual agent."
the credit binding, how to record the record of loss of trust, but also directly affect the drug enterprise's subsequent marketing behavior.
but to some extent, the National Health Insurance Administration has built a skynet in the collection of misin confidence in pharmaceutical companies, allowing all pharmaceutical companies to "avoid it".
Collecting and recording the information of the loss of trust includes the information that the pharmaceutical enterprise has voluntarily reported, the information disclosed or generated by the collection and recording department, and the information of the misconceited behavior of the pharmaceutical enterprise in pricing, bidding, performance, marketing and so on through monitoring and receiving the report in the daily operation of the centralized purchasing organization.
3, loss of trust, how to deal with? The provinces shall adopt disposal measures according to the credit rating results.
medical enterprises rated as "general" for failure of trust shall be given written warnings by provincial centralized purchasing agencies.
For pharmaceutical enterprises rated as "moderate" for loss of trust, in addition to warnings, credit rating results should be marked in the platform information of pharmaceutical enterprises or related pharmaceutical products, and when medical institutions place orders for the purchase of drugs or medical supplies produced or distributed by the enterprise, the purchase object should be automatically prompted for the risk of non-trust.
For pharmaceutical enterprises rated as "serious", in addition to warning and warning of risks, the enterprise involved in the case of drugs or medical supplies should be restricted or suspended, bidding or distribution qualifications, restrictions or suspension period according to the pharmaceutical enterprise credit repair behavior and results of timely adjustment.
If a pharmaceutical enterprise restricts or suspends the qualification for the registration, bidding or distribution of a specific drug or medical consumables, it shall be the responsibility to expressly consume for the mis-confidence of the drug or medical consumables, and the conversion according to the 5.3.3 rating method shall be sufficient to constitute a "serious" case of disinreponance.
For pharmaceutical enterprises rated as "particularly serious", in addition to warning and warning of risks, the enterprise shall be restricted or suspended all drugs and medical supplies hanging network, bidding or distribution qualifications, limit or suspension period according to the pharmaceutical enterprise credit repair behavior and results of timely adjustment.
for pharmaceutical enterprises rated as "serious" and "particularly serious" for their failure of trust, the provincial centralized procurement agencies regularly disclose the results and relevant information of the enterprise's rating to the public and accept social supervision.
This means that when the credit data of drug recruitment accumulates to a certain extent, centralized purchasing agencies will give priority to pharmaceutical companies with better credit records to conduct transactions, but also to the main body of disrepair, poor records of pharmaceutical companies to refuse transactions, improve the trading conditions of discipline, serious disinception enterprises are kicked out of the public procurement market.
, the State Health Insurance Administration officially issued the "guidance on the establishment of medical prices and credit evaluation system" medical insurance issued (2020) No. 34. the
document points out that the State Health Insurance Administration has established a list of medical prices and the list of matters of breach of trust, implemented dynamic adjustments, and included in the list of non-trust matters mainly include the payment of rebates or other improper benefits in the purchase and sale of medicines ("pharmaceutical commercial bribery"), tax-related violations, the implementation of monopoly acts, improper price behavior, disturbing the centralized procurement order, malicious breach of contract agreements and other acts contrary to good faith.
with the pharmaceutical recruitment credit evaluation operating norms in the country, pharmaceutical enterprises will be included in the purchase and sale of all-round monitoring, and affect the follow-up marketing of enterprises.




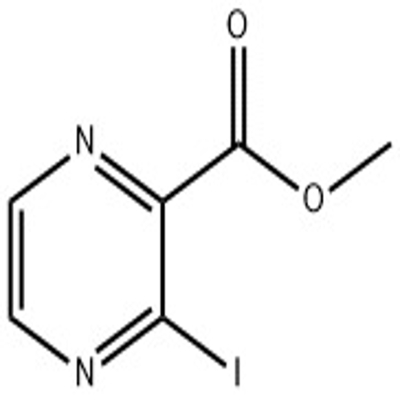
![6-Chloro-1H-pyrazolo[3,4-b]pyrazine](https://file.echemi.com/fileManage/upload/goodpicture/20210820/m20210820163301650.jpg)