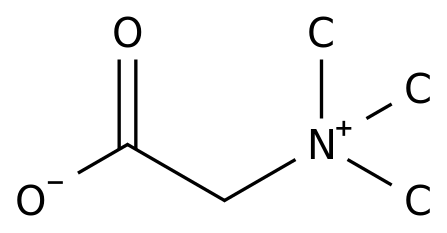
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
order of the Ministry of Agriculture of the People's Republic of China
XNq2013
XNq"Measures for the Administration of Veterinary And Over-the-Counter Drugs" was approved by the 7th Executive Meeting of the Ministry of Agriculture on August 1, 2013 and is hereby promulgated, effective March 1, 2014.
XNq . Annex: "
XNqMinister Han Changfu
XNqSeptember 11, 2013
XNq
The Management Measures for Veterinary And Prescription Drugs
XNq
Article 1
to strengthen the supervision and management of veterinary drugs, promote clinical rational use of veterinary drugs, and safeguard the safety of animal products, according to the Regulations on the Administration of Veterinary Medicines.
XNq
Article 2
The State shall implement the classification and management of veterinary drugs, which are classified into veterinary and over-the-counter drugs according to the safety and risk of veterinary drugs.
XNq .Veterinary prescription drugs are veterinary drugs that can be purchased and used on the basis of veterinary prescriptions.
XNq .Veterinary over-the-counter medicine s is a veterinary drug that can be purchased and used in accordance with the instructions without the need for a veterinary prescription note.
XNq .The list of prescription drugs for veterinary use is developed and published by the Department of Agriculture. Veterinary drugs other than the veterinary prescription drug catalogue are over-the-counter for veterinary use.
XNq
Article 3
the Department of Agriculture is responsible for the management of prescription and over-the-counter drugs for veterinary use throughout the country.
XNq .The veterinary administrative department of the local people's government at or above the county level shall be responsible for the supervision and management of veterinary and over-the-counter drugs in the administrative area, and the specific work may be entrusted to the law enforcement agencies to which they belong.
XNq
Article 4
the label and instructions for veterinary prescription drugs shall be marked with the words "Veterinary prescription drugs" and the labels and instructions for veterinary over-the-counter drugs shall be marked with the words "Over-the-Counter Drug for Veterinary Use".
XNq .The words of the preceding paragraph should be marked in the upper right corner of the label and instruction manual in a red form, the background should be white, the font size according to the actual needs of the setting, but must be eye-catching and clear.
Article 5 of the
of XNq
the enterprise of veterinary medicine shall track the safety and effectiveness of veterinary drugs produced by the enterprise and, if found unsuitable for management under veterinary over-the-counter drugs, report to the Ministry of Agriculture in a timely manner.
XNq .If an veterinary drug operator, an animal medical treatment institution, a trade association or other organization or individual discovers that an over-the-counter veterinary drug has the circumstances specified in the preceding paragraph, it shall report to the local veterinary administrative department.article 6 of the
XNq
veterinary drug operators shall hang or post a warning that "prescription drugs for veterinary use must be purchased with a veterinary prescription" in a prominent place in the business premises.
XNq .Veterinary drug operators shall be divided or placed in subcabinets for veterinary prescription drugs and veterinary over-the-counter sass. Veterinary prescription drugs may not be sold on an open rack option.Article 7 of the
XNq
the use of veterinary prescription drugs may be traded on the basis of veterinary prescriptions, except in the following cases:
XNq(i) import and export of veterinary prescription drugs;
XNq(2) to sell prescription veterinary drugs to animal medical institutions, scientific research units, animal disease prevention and control institutions and other veterinary drug production enterprises and operators;
XNq(3) to sell veterinary prescription drugs to animal farms (breeding communities), zoos, experimental animal feeding farms, etc. with full-time licensed veterinarians registered in accordance with the Measures for the Administration of Practicing Veterinary Medicines.article 8 of the
XNq
veterinary prescription notes shall be issued by a legally registered practicing veterinarian in accordance with the scope of his or her registered practice.Article 9 of the
XNq
veterinary prescription notes shall include the following:
XNq(i) the name of the owner or the name of the animal farm;
XNq(ii) animal species, age (day) age, weight and quantity;
XNq (iii) diagnostic results;
XNq (iv) generic name, specification, quantity, usage, dosage and duration of the drug;
XNq (v) the prescription date and the prescription veterinary registration number and seal.
XNq . Prescription notes are in a three-way way, the first joint shall be kept by an animal clinic or a licensed veterinarian who prescribes prescription drugs, the second joint shall be kept by the veterinary drug operator, and the third joint shall be kept by the owner or animal farm. Prescriptions issued by full-time practicing veterinarians in animal farms (breeding communities), zoos, experimental animal feeding farms and other units shall be kept by the units of full-time practicing veterinarians.
XNq . Prescription notes should be kept for more than two years. article 10 of the
XNq
veterinary drug operators shall examine veterinary prescription notes, separately establish records of the purchase and sale of veterinary prescription drugs, and keep them for more than two years.
XNq
Article 11
prescription drugs for veterinary use shall be used in accordance with the matters contained in the prescription notes. Article 12
XNq
Rural veterinarians shall use veterinary drugs in accordance with the "Basic Medicine Stakes Directory for Rural Veterinary Medicines" formulated and published by the Ministry of Agriculture. Article 13 of the
XNq
the production, sale and use of special drugs such as veterinary narcotic drugs, psychotropic drugs and toxic drugs shall also comply with the relevant provisions of the State. Article 14 of the
XNq
who violates the provisions of Article 4 of these Measures shall be punished in accordance with the provisions of Article 60, paragraph 2, of the Regulations on the Administration of Veterinary Medicines. Article 15 of the
XNq
, in violation of the provisions of these Measures, sells, purchases or uses prescription drugs for veterinary use without a registered licensed veterinary surgeon, shall be punished in accordance with the provisions of Article 66 of the Regulations on the Administration of Veterinary Medicines. Article 16 of the
XNq
in violation of the provisions of these Measures, if any of the following circumstances are made, the penalty shall be imposed in accordance with the provisions of Article 59 (1) of the Regulations on the Administration of Veterinary Medicines:
XNq (1) If the veterinary drug operator fails to hang or post a prompt in an obvious position in the business premises;
XNq (2) Veterinary prescription drugs and veterinary over-the-counter drugs are not divided or placed in subcabinets;
XNq (iii) veterinary prescription drugs sold by open rack optional manner;
XNq (4) veterinary prescription notes and veterinary prescription drug purchase and sale records are not kept as prescribed. Article 17 of the
XNq
, if a
violates other provisions of these Measures, he shall be punished in accordance with the relevant provisions of the Animal Epidemic Prevention Law of the People's Republic of China and the Regulations on the Administration of Veterinary Medicines. Article 18 of the
XNq
these Measures shall come into effect on 1 March 2014.
XNq
Order of the Ministry of Agriculture of the People's Republic of China
XNq 2013
XNq "Measures for the Administration of Veterinary Prescription and Over-The-Prescription Drugs" was approved by the 7th Executive Meeting of the Ministry of Agriculture on August 1, 2013 and is hereby issued, effective From March 1, 2014.
XNq . Annex: "
XNq Minister Han Changfu
XNq September 11, 2013
XNq
The Management Measures for Veterinary And Prescription Drugs
XNq
Article 1
to strengthen the supervision and management of veterinary drugs, promote clinical rational use of veterinary drugs, and safeguard the safety of animal products, according to the Regulations on the Administration of Veterinary Medicines.
XNq
Article 2
The State shall implement the classification and management of veterinary drugs, which are classified into veterinary and over-the-counter drugs according to the safety and risk of veterinary drugs.
XNq . Veterinary prescription drugs are veterinary drugs that can be purchased and used on the basis of veterinary prescriptions.
XNq . Veterinary over-the-counter medicine s is a veterinary drug that can be purchased and used in accordance with the instructions without the need for a veterinary prescription note.
XNq . The list of prescription drugs for veterinary use is developed and published by the Department of Agriculture. Veterinary drugs other than the veterinary prescription drug catalogue are over-the-counter for veterinary use.
XNq
Article 3
the Department of Agriculture is responsible for the management of prescription and over-the-counter drugs for veterinary use throughout the country.
XNq . The veterinary administrative department of the local people's government at or above the county level shall be responsible for the supervision and management of veterinary and over-the-counter drugs in the administrative area, and the specific work may be entrusted to the law enforcement agencies to which they belong.
XNq
Article 4
the label and instructions for veterinary prescription drugs shall be marked with the words "Veterinary prescription drugs" and the labels and instructions for veterinary over-the-counter drugs shall be marked with the words "Over-the-Counter Drug for Veterinary Use".
XNq . The words of the preceding paragraph should be marked in the upper right corner of the label and instruction manual in a red form, the background should be white, the font size according to the actual needs of the setting, but must be eye-catching and clear.
Article 5 of the
of XNq
the enterprise of veterinary medicine shall track the safety and effectiveness of veterinary drugs produced by the enterprise and, if found unsuitable for management under veterinary over-the-counter drugs, report to the Ministry of Agriculture in a timely manner.
XNq . If an veterinary drug operator, an animal medical treatment institution, a trade association or other organization or individual discovers that an over-the-counter veterinary drug has the circumstances specified in the preceding paragraph, it shall report to the local veterinary administrative department. article 6 of the
XNq
veterinary drug operators shall hang or post a warning that "prescription drugs for veterinary use must be purchased with a veterinary prescription" in a prominent place in the business premises.
XNq . Veterinary drug operators shall be divided or placed in subcabinets for veterinary prescription drugs and veterinary over-the-counter sass. Veterinary prescription drugs may not be sold on an open rack option. Article 7 of the
XNq
the use of veterinary prescription drugs may be traded on the basis of veterinary prescriptions, except in the following cases:
XNq (i) import and export of veterinary prescription drugs;
XNq (2) to sell prescription veterinary drugs to animal medical institutions, scientific research units, animal disease prevention and control institutions and other veterinary drug production enterprises and operators;
XNq (3) to sell veterinary prescription drugs to animal farms (breeding communities), zoos, experimental animal feeding farms, etc. with full-time licensed veterinarians registered in accordance with the Measures for the Administration of Practicing Veterinary Medicines. article 8 of the
XNq
veterinary prescription notes shall be issued by a legally registered practicing veterinarian in accordance with the scope of his or her registered practice. Article 9 of the
XNq
veterinary prescription notes shall include the following:
XNq (i) the name of the owner or the name of the animal farm;
XNq (ii) animal species, age (day) age, weight and quantity;
XNq (iii) diagnostic results;
XNq (iv) generic name, specification, quantity, usage, dosage and duration of the drug;
XNq (v) the prescription date and the prescription veterinary registration number and seal.
XNq . Prescription notes are in a three-way way, the first joint shall be kept by an animal clinic or a licensed veterinarian who prescribes prescription drugs, the second joint shall be kept by the veterinary drug operator, and the third joint shall be kept by the owner or animal farm. Prescriptions issued by full-time practicing veterinarians in animal farms (breeding communities), zoos, experimental animal feeding farms and other units shall be kept by the units of full-time practicing veterinarians.
XNq . Prescription notes should be kept for more than two years. article 10 of the
XNq
veterinary drug operators shall examine veterinary prescription notes, separately establish records of the purchase and sale of veterinary prescription drugs, and keep them for more than two years.
XNq
Article 11
prescription drugs for veterinary use shall be used in accordance with the matters contained in the prescription notes. Article 12
XNq
Rural veterinarians shall use veterinary drugs in accordance with the "Basic Medicine Stakes Directory for Rural Veterinary Medicines" formulated and published by the Ministry of Agriculture.
XNq
Article 13
veterinary narcotics.