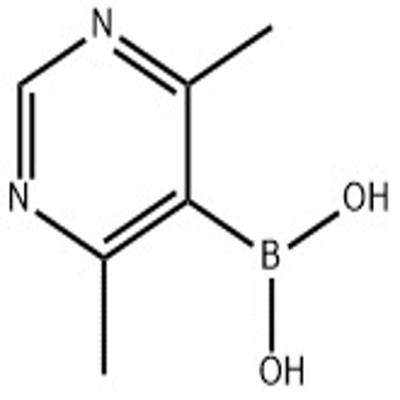
-
Categories
-
Pharmaceutical Intermediates
-
Active Pharmaceutical Ingredients
-
Food Additives
- Industrial Coatings
- Agrochemicals
- Dyes and Pigments
- Surfactant
- Flavors and Fragrances
- Chemical Reagents
- Catalyst and Auxiliary
- Natural Products
- Inorganic Chemistry
-
Organic Chemistry
-
Biochemical Engineering
- Analytical Chemistry
- Cosmetic Ingredient
-
Pharmaceutical Intermediates
Promotion
ECHEMI Mall
Wholesale
Weekly Price
Exhibition
News
-
Trade Service
On November 17, Nanjing Sheng and submitted a class 1 new drug and Lebwe tablets to the market application was accepted by CDE for the treatment of chronic hepatitis C.
and Lebwe are the first NS5B inhibitors independently designed and developed by Sanhe Pharmaceuticals, with strong liver targeting ability, high antiviral activity, and non-clinical research data confirm that the in-body antiviral activity of SH229 gene type 1-6 HCV is 2-3 times that of sophosphorus buvir.
the results of a Phase II clinical study (CTR20182539) at the annual meeting of the European Society of Hepatology (EASL) in August this year.
the study evaluated the safety and effectiveness of SH229 combined with Daratawe in Chinese patients treating pangene HCV infection.
This is an open-label clinical study in which Chinese patients infected with HCV were randomly divided into three queues at a scale of 1:1:1, receiving 12 weeks of Daratawe (60mg, 1 time/day) combined with SH229 400mg (queue A), 600mg (queue B) or 800mg (queue C).
factors include HCV genotype and cirrhosis.
end point of this study was the virus sustained response rate (SVR12) 12 weeks after treatment.
results showed that among the 124 patients assessed by SVR12, SVR12 of patients treated with type 1/2 HCV infection reached 100%, and the treatment effect of patients with type 3/6 HCV infection was also better, of which SVR12 of patients with type 6 HCV infection reached 93.3%.
safety, all subjects were well-to-do and did not treat the relevant SAE or AE suspension events.




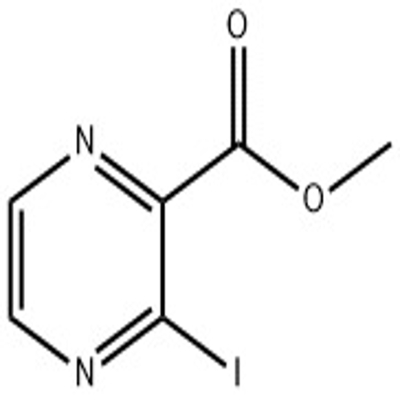
![6-Chloro-1H-pyrazolo[3,4-b]pyrazine](https://file.echemi.com/fileManage/upload/goodpicture/20210820/m20210820163301650.jpg)